Abstract
Background
COPD is associated with cardiovascular disease (CVD), and coronary artery calcification (CAC) provides additional prognostic information. With increasing use of nongated CT scans in clinical practice, this study hypothesized that the visual Weston CAC score would perform as well as the Agatston score in predicting prevalent and incident coronary artery disease (CAD) and CVD in COPD.
Methods
CAC was measured by using Agatston and Weston scores on baseline CT scans in 1,875 current and former smokers enrolled in the Genetic Epidemiology of COPD (COPDGene) study. Baseline cardiovascular disease and incident cardiac events on longitudinal follow-up were recorded. Accuracy of the CAC scores was measured by using receiver-operating characteristic analysis, and Cox proportional hazards analyses were used to estimate the risk of incident cardiac events.
Results
CAD was reported by 133 (7.1%) subjects at baseline. A total of 413 (22.0%) and 241 (12.9%) patients had significant CAC according to the Weston (≥ 7) and Agatston (≥ 400) scores, respectively; the two methods were significantly correlated (r = 0.84; P < .001). Over 5 years of follow-up, 127 patients (6.8%) developed incident CVD. For predicting prevalent CAD, c-indices for the Weston and Agatston scores were 0.78 and 0.74 and for predicting incident CVD, they were 0.62 and 0.61. After adjustment for age, race, sex, smoking pack-years, FEV1, percent emphysema, and CT scanner type, a Weston score ≥ 7 was associated with time to first acute coronary event (hazard ratio, 2.16 [95% CI, 1.32 to 3.53]; P = .002), but a Agatston score ≥ 400 was not (hazard ratio, 1.75 [95% CI, 0.99-3.09]; P = .053).
Conclusions
A simple visual score for CAC performed well in predicting incident CAD in smokers with and without COPD.
Trial Registry
ClinicalTrials.gov; No.: NCT00608764; URL: www.clinicaltrials.gov.
Key Words: cardiovascular disease, COPD, coronary calcification
Abbreviations: CAC, coronary artery calcification; CAD, coronary artery disease; CVD, cardiovascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; HU, Hounsfield units
COPD is the third leading cause of death in the United States and is associated with significant morbidity. Although primarily a disease that involves chronic inflammation of the lung, COPD is now recognized as a multisystem disease that is associated with accelerated atherosclerosis and cardiovascular disease.1 Cardiovascular disease accounts for the majority of mortality in mild to moderate COPD.1, 2 All manifestations of cardiovascular disease, including coronary artery disease (CAD), ischemic stroke, peripheral arterial disease, and the need for intervention, are considerably greater in subjects with COPD after adjustment for shared risk factors such as age and cigarette smoking.3, 4, 5 Unfortunately, cardiovascular disease is silent and asymptomatic in a majority of patients, and this is exacerbated in those with COPD in whom symptoms can be ascribed to underlying lung disease. Numerous approaches have been used to stratify patients at risk for adverse cardiovascular events, including scoring systems such as the Framingham coronary heart disease risk score.6 Noninvasive surrogates for the presence of cardiovascular disease such as coronary artery calcification (CAC) on CT imaging have been shown to offer risk assessment over that afforded by the scoring systems in the general population.7, 8 However, these scoring systems have not been specifically tested in populations with COPD.
CT imaging of the chest is performed frequently and accounts for > 20% of CT scans conducted in the United States.9 This number is expected to increase with the advent of low-dose screening for lung cancer in smokers.10 This approach offers an opportunity to easily screen and test for cardiovascular disease in patients at risk. CAC is traditionally measured by using the semi-automated Agatston score on electrocardiographically gated CT scans, but standard and low-dose nongated scans have been shown to be reliable in scoring the presence of CAC in patients with COPD.11, 12 Although the Agatston score performs well in the prediction of existing and incident cardiovascular disease and events, limited studies are available that assess CAC using this method in COPD.13, 14, 15 Only one small study found a relationship between the presence of CAC and incident and recurrent cardiovascular events in subjects with COPD.15 The applicability of the Agatston score is further limited by the need for special software and often a separate work station.16 With increasing use of nongated CT scans in clinical practice, we hypothesized that a simple visual score (Weston) would perform as well as the Agatston score in predicting prevalent CAD and incident cardiovascular disease in smokers with and without COPD.17
Subjects and Methods
We analyzed subjects enrolled in a large multicenter cohort study (Genetic Epidemiology of COPD [COPDGene]) with current and former smokers aged 45 to 80 years. The details of this study have been previously published.18 Participants with and without COPD were included, and those with known lung disease other than COPD and asthma were excluded. The diagnosis of COPD was made with postbronchodilator spirometry by using the ratio of FEV1 to FVC of < 0.70.19 Participants with FEV1/FVC ≥ 0.70 but with FEV1 < 80% predicted were deemed to have Global Initiative for Chronic Obstructive Lung Disease (GOLD) COPD unclassifiable disease or preserved ratio-impaired spirometry.20 Those without airflow obstruction on spirometry were categorized as smoking control subjects. Demographic data were recorded at enrollment, and prevalent cardiovascular disease was recorded as patient-reported physician-diagnosed conditions. Cardiovascular disease was recorded to be present if participants had one or more of CAD, ischemic cerebrovascular disease, and peripheral arterial disease. Participants at seven centers who had visual CAC measured on-site were included in the current analyses.
Participants were prospectively followed up for approximately 5 years by contacting them every 3 to 6 months using an automated telephone system, by Internet data collection, or by research coordinators.21 Acute coronary events were recorded for time intervals rounded off to the nearest month. Information was obtained on incident diagnosis of cardiovascular disease, including procedures such as percutaneous cardiac interventions and coronary artery bypass grafting, and recorded as present or absent at their follow-up visit for phase two of the COPDGene study at approximately 5 years after enrollment. We used 3D Slicer software (www.airwayinspector.org) to measure percent emphysema on inspiratory images.18 Using density mask analyses, emphysema was quantified as the percentage of voxels with attenuation less than –950 Hounsfield units (HU). Written informed consent was obtained from all participants prior to study enrollment, and the COPDGene study was approved by the institutional review boards of all participating centers (F070712014).
Measurement of Coronary Artery Calcification
Thin-section helical CT scans were performed in all participants, and inspiratory scans were used for measurement of CAC using two methods. The scans were obtained with an imaging protocol with the following details: collimation, 0 to 5 mm; tube voltage, 120 kV; tube current, 200 mAs; gantry rotation time, 0.5 s; and pitch, 1.1. The images were reconstructed with a standard kernel with a slice thickness of 0.75 mm and a reconstruction interval of 0.5 mm. First, the Agatston score was measured by using standard software (Heartbeat-CS, Extended Brilliance Workspace, Philips Medical System, Best, the Netherlands).22 To quantify CAC, a threshold was set for calcific lesions involving three contiguous voxels that had a CT density of 130 HU with an area ≥ 1 mm2. As described by Agatston, a density factor was determined for each area of CAC: 1 = 130 to 199; 2 = 200 to 299; 3 = 300 to 399; and 4 = ≥ 400 HU. The lesion score for each area of CAC was calculated by multiplying the area of calcification by the density factor. The total Agatston score was then determined by summing individual lesion scores from each of four anatomical sites (left main, left anterior descending, circumflex, and right coronary arteries). An Agatston score ≥ 400 was defined as clinically significant.23
At each of the seven centers, experienced radiologists also visually analyzed the coronary arteries to calculate the visual Weston score.17 The CT images were assessed visually by using mediastinal soft tissue window settings (window width: 400; window length: 40). The Weston score assigns values based on visual estimates for the presence and degree of calcification in each of the major coronary arteries as follows: 0, no visually detected calcium; 1, only a single high-density pixel detected; 3, calcium dense enough to cause a blooming artifact; and 2, for calcium intermediate and between 1 and 3. All readers were blinded to the results of the Agatston scores and participants’ demographic and clinical data. Based on the original description correlating Weston scores with Agatston scores, Weston scores ≥ 7 were defined as clinically significant.17
Statistical Analyses
All values are expressed as mean ± SD. The correlation between Agatston and Weston CAC scores was analyzed by using the nonparametric Spearman test. Intra-observer and interobserver variability was tested by using intraclass correlation coefficients. After categorizing participants into groups based on Agatston scores ≥ 400 and Weston scores ≥ 7, independent t tests and χ2 tests were used to compare differences between the groups, including differences in prevalent and incident cardiovascular disease between groups. Receiver-operating characteristic curves were used to assess the accuracy of the two scores in predicting prevalent CAD and incident cardiovascular disease. The risk of acute coronary event on follow-up was assessed by time to first event using Cox proportional hazards models, with adjustment for age, race, sex, smoking pack-years, FEV1, percent emphysema, and CT scanner type. Associations between COPD parameters and CAC were tested with univariate and multivariable linear regression analyses. All tests of significance were two-tailed, with statistical significance deemed to be at an alpha level of 0.05. All analyses were performed by using SPSS version 24.0 (IBM SPSS Statistics, IBM Corporation) and R statistical software version 3.2 (R Foundation for Statistical Computing).
Results
Demographic Characteristics
Visual CAC was measured in 1,913 participants at seven centers. Of these, 10 were excluded due to unacceptable spirometry data and 28 due to unavailable Agatston scores; the final sample size was 1,875. The mean age of the cohort was 60.7 ± 8.1 years; 957 (51%) were male, and 480 (25.6%) were of African-American race. Participants had a significant cigarette smoking burden, with mean pack-years of 43.4 ± 23.8; 869 (46.3%) were active smokers at the time of enrollment. A total of 1,017 (54.2%) had COPD, and participants spanned the spectrum of severity of airflow obstruction with 858 (45.8%), 184 (9.8%), 379 (20.2%), 179 (9.5%), and 42 (2.2%) having GOLD stages 0, 1, 2, 3, and 4, respectively. A total of 233 patients (12.4) had GOLD unclassified or preserved ratio-impaired spirometry.
A large proportion of participants had significant cardiovascular comorbidities. The frequency of diabetes mellitus, hypertension, and hyperlipidemia was 234 (12.5%), 820 (43.7%), and 798 (42.6%), respectively. A total of 133 (7.1%) had CAD at enrollment; 40 (2.1%) had peripheral arterial disease; and 46 (2.5%) had a history of ischemic stroke. The cumulative frequency of cardiovascular disease at baseline was 198 (10.6%); 103 (5.5%) had undergone percutaneous coronary interventions, and 41 (2.2%) had undergone coronary artery bypass grafting.
Coronary Artery Calcification
The intra-observer and interobserver agreement for scoring visual CAC was excellent (intraclass correlation coefficient: 0.98 [95% CI, 0.95 to 0.99], P < .001 and 0.97 [95% CI, 0.94-0.99], P < .001, respectively). The median Weston score was 3 (interquartile range, 0-6). A total of 507 (27.0%) subjects had 0 CAC on visual analyses, and 413 (22%) had a Weston score ≥ 7. The median Agatston score was 31 (interquartile range, 0-191). A total of 581 (31%) subjects had 0 Agatston CAC. There were 659 (35.1%) subjects with CAC of at least 100, 306 (16.3%) with CAC ≥ 300, and 241 (12.9%) with CAC ≥ 400.
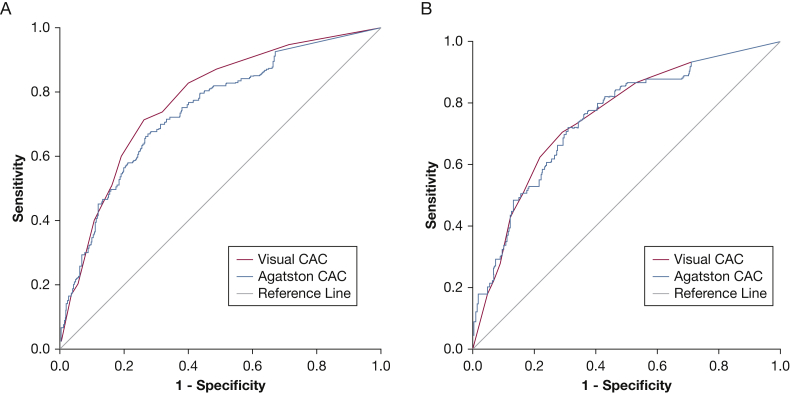
Table 1 presents a comparison of baseline demographic characteristics, comorbidities, and cardiovascular disease in those with significant CAC according to the two methods. The Agatston and Weston scores correlated significantly (Spearman r = 0.84; P < .001). On receiver-operating characteristic analyses, the accuracy of CAC was comparable for prevalent CAD at baseline according to the Agatston and Weston scores: c-indices of 0.74 (95% CI, 0.70-0.79; P < .001) and 0.78 (95% CI, 0.74-0.82; P < .001), respectively (Fig 1A). In those with COPD, the accuracy was comparable for the two scores: c-indices of 0.75 (95% CI, 0.70-0.80; P < .001) for the Agatston score and 0.76 (95% CI, 0.70-0.81; P < .001) for the Weston score (Fig 1B).
Table 1.
Comparison of Baseline Demographic Characteristics and Cardiovascular Disease Data According to CAC Scores
| Variable | Agatston CAC |
Visual CAC |
||||
|---|---|---|---|---|---|---|
| < 400 (n = 1,634) | ≥ 400 (n = 241) | P Value | < 7 (n = 1,462) | ≥ 7 (n = 413) | P Value | |
| Age, y | 60.0 ± 8.0 | 65.6 ± 7.2 | < .001 | 59.6 ± 8.0 | 64.8 ± 7.3 | < .001 |
| Male sex | 783 (47.9) | 174 (72.2) | < .001 | 687 (47.0) | 270 (65.4) | < .001 |
| Race, non-Hispanic white | 1,191 (72.9) | 204 (84.6) | < .001 | 1,044 (71.4) | 351 (85.0) | < .001 |
| BMI, kg/m2 | 29.1 ± 6.0 | 29.8 ± 5.6 | .106 | 29.2 ± 6.2 | 29.2 ± 5.3 | .971 |
| Smoking pack-years | 42.2 ± 23.3 | 51.6 ± 25.6 | < .001 | 42.0 ± 22.9 | 26.5 ± 22.6 | < .001 |
| Current smoker | 786 (48.1) | 83 (34.4) | < .001 | 715 (48.9) | 154 (37.3) | < .001 |
| Diabetes mellitus | 186 (11.4) | 48 (19.9) | < .001 | 163 (11.1) | 71 (17.2) | .001 |
| Hypertension | 675 (41.3) | 145 (60.2) | < .001 | 584 (39.9) | 236 (57.1) | < .001 |
| Hyperlipidemia | 658 (40.3) | 140 (58.1) | < .001 | 568 (38.9) | 230 (55.7) | < .001 |
| Coronary artery disease | 84 (5.1) | 49 (20.3) | < .001 | 53 (3.6) | 80 (19.4) | < .001 |
| Ischemic cerebrovascular disease | 34 (2.1) | 12 (5.0) | .007 | 29 (2.0) | 17 (4.1) | .013 |
| Peripheral arterial disease | 27 (1.7) | 13 (5.4) | < .001 | 22 (1.5) | 18 (4.4) | < .001 |
| Percutaneous coronary intervention | 68 (4.2) | 35 (14.5) | < .001 | 36 (2.5) | 67 (16.2) | < .001 |
| Coronary artery bypass grafting | 24 (1.5) | 17 (7.1) | < .001 | 10 (0.7) | 31 (7.5) | < .001 |
| GOLD stage | .211 | < .001 | ||||
| 0 | 764 (46.8) | 94 (39.0) | 699 (47.8) | 159 (38.5) | ||
| 1 | 159 (9.7) | 25 (10.4) | 138 (9.4) | 46 (11.1) | ||
| 2 | 317 (19.4) | 62 (25.7) | 266 (18.2) | 113 (27.4) | ||
| 3 | 155 (9.5) | 24 (10.0) | 132 (9.0) | 47 (11.4) | ||
| 4 | 36 (2.2) | 6 (2.5) | 32 (2.2) | 10 (2.4) | ||
| PRISm | 203 (12.4) | 30 (12.4) | 195 (13.3) | 38 (9.2) | ||
| FEV1, L | 2.33 ± 0.83 | 2.34 ± 0.82 | .854 | 2.35 ± 0.82 | 2.28 ± 0.84 | .164 |
| FEV1 % predicted | 80.1 ± 22.4 | 77.5 ± 21.2 | .090 | 80.5 ± 22.1 | 77.0 ± 22.6 | .005 |
| FVC, L | 3.37 ± 0.96 | 3.51 ± 0.96 | .028 | 3.37 ± 0.96 | 3.47 ± 0.98 | .042 |
| FVC % predicted | 89.1 ± 16.5 | 87.8 ± 16.3 | .244 | 89.0 ± 16.3 | 88.7 ± 16.9 | .741 |
| FEV1/FVC | 0.69 ± 0.14 | 0.66 ± 0.14 | .009 | 0.69 ± 0.14 | 0.65 ± 0.14 | < .001 |
| % Emphysema | 5.4 ± 7.7 | 6.4 ± 8.1 | .069 | 5.1 ± 7.6 | 6.8 ± 8.2 | < .001 |
Data are presented as mean ± SD or No. (%). CAC = coronary artery calcification; GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
Figure 1.
A, The accuracy of coronary artery calcification (CAC) for prevalent coronary artery disease at baseline according to the Agatston and Weston scores was comparable using receiver-operating characteristic analyses: c-indices of 0.74 (95% CI, 0.70-0.79; P < .001) and 0.78 (95% CI, 0.74-0.82; P < .001), respectively. B, In those with COPD, the accuracy was comparable for the two scores: c-indices of 0.75 (95% CI, 0.70-0.80; P < .001) for the Agatston score and 0.76 (95% CI, 0.70-0.81; P < .001) for the Weston score.
Table 2 shows that cardiovascular comorbidity at baseline was significantly greater in those with COPD compared with the smoking control subjects. The prevalence of cumulative cardiovascular disease was 134 (13.2%) in those with COPD compared with 64 (7.5%) in those without COPD (P < .001). Agatston scores ≥ 400 were present in more patients with COPD (147 [14.5%]) than in control subjects (94 [11.0%]; P = .024). There was a similar difference in the prevalence of Weston scores ≥ 7: 254 (25%) vs 159 (18.5%), P = .001. Based on Agatston and Weston thresholds, there were 192 (10.2%) and 333 (17.8%) participants with undiagnosed CAD, respectively. Compared with control subjects, more participants with COPD had undiagnosed CAD based on both Agatston (80 [9.3%] vs 112 [11.0%]; P = .007) and Weston (199 [19.6%] vs 134 [15.6%]; P = .001) thresholds.
Table 2.
Comparison of Baseline Demographic Characteristics in Participants With and Without COPD
| Characteristic | Subjects With COPD (n = 1,017) | Smoking Control Subjects (n = 858) | P Value |
|---|---|---|---|
| Age, y | 61.8 ± 8.0 | 59.5 ± 8.2 | < .001 |
| Male sex | 528 (51.9) | 429 (50.0) | .408 |
| Race, non-Hispanic white | 762 (74.9) | 633 (73.8) | .570 |
| BMI, kg/m2 | 29.1 ± 6.4 | 29.2 ± 5.5 | .772 |
| Smoking pack-years | 48.0 (25.3) | 37.9 (20.7) | < .001 |
| Current smoker | 476 (46.8) | 393 (45.8) | .665 |
| Diabetes mellitus | 126 (12.4) | 108 (12.6) | .897 |
| Hypertension | 475 (46.7) | 345 (40.2) | .005 |
| Hyperlipidemia | 430 (42.3) | 368 (42.9) | .790 |
| Coronary artery disease | 89 (8.8) | 44 (5.1) | .002 |
| Ischemic cerebrovascular disease | 34 (3.3) | 12 (1.4) | .007 |
| Peripheral arterial disease | 29 (2.9) | 11 (1.3) | .019 |
| Percutaneous coronary intervention | 67 (6.6) | 36 (4.2) | .024 |
| Coronary artery bypass grafting | 26 (2.6) | 15 (1.7) | .233 |
| Agatston CAC | |||
| ≥ 100 | 396 (38.9) | 263 (30.7) | < .001 |
| ≥ 300 | 197 (19.4) | 109 (12.7) | < .001 |
| ≥ 400 | 147 (14.5) | 94 (11.0) | .024 |
| Weston CAC ≥ 7 | 254 (25.0) | 159 (18.5) | < .001 |
Data are presented as mean ± SD or No. (%). See Table 1 legend for expansion of abbreviation.
Follow-up
Participants were prospectively followed up for a median duration of 5.8 years (interquartile range, 4.9-6.3 years). At the 5-year return visit, an additional 127 (6.8%) participants had a new diagnosis of cardiovascular disease. A greater number of patients with COPD developed incident cardiovascular disease (80 [8.1%]) compared with control subjects (47 [5.7%]; P = .041). For predicting incident cardiovascular disease, both measures performed modestly, with c-indices of 0.61 for the Agatston score (95% CI, 0.56-0.66; P < .001) and 0.62 for the Weston score (95% CI, 0.57-0.68; P < .001). Compared with participants with known CAD who developed additional CVD events, both Weston scores ≥ 7 and Agatston scores ≥ 400 identified patients with undiagnosed CAD who developed incident CVD (71 [5.2%] vs 33 [10.2%]; P = .001 for Weston scores; 79 [5.3%] vs 25 [13.2%] for Agatston scores; P < .001).
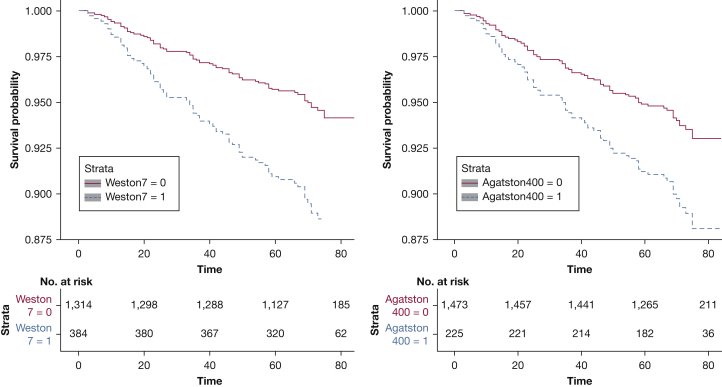
We also compared the utility of the two scores in estimating time to first acute coronary event on follow-up. Compared with Agatston scores < 400, a score ≥ 400 was associated with a shorter time to first event (unadjusted hazards ratio [HR], 2.18 [95% CI, 1.30-3.65]; P = .003) but not after adjustment for age, race, sex, smoking pack-years, FEV1, percent emphysema, and CT scanner type (HR, 1.75 [95% CI, 0.99-3.09]; P = .053). In contrast, compared with a Weston score < 7, a score ≥ 7 was associated with a shorter time to first coronary event (unadjusted HR, 2.40 [95% CI, 1.53-3.76], P < .001; adjusted HR, 2.16 [95% CI, 1.32-3.53], P = .002) (Fig 2).
Figure 2.
Kaplan-Meier curves comparing the Weston visual score with the Agatston score for acute coronary event-free follow-up. After adjustment for age, race, sex, smoking pack-years, FEV1, percent emphysema, and CT scanner type, Weston scores ≥ 7 compared with scores < 7 were associated with a shorter time to first coronary event (adjusted hazard ratio: 2.16 [95% CI, 1.32-3.53]; P = .002). In contrast, compared with Agatston scores < 400, a score ≥ 400 was not associated with a shorter time to first event (adjusted HR, 1.75 [95% CI, 0.99-3.09]; P = .053).
Association With COPD Parameters
After multivariable adjustment for age, race, sex, smoking pack-years, and CT scanner type, FEV1 was inversely associated with Weston CAC (adjusted beta co-efficient: –0.264 [95% CI, –0.481 to –0.046]; P = .017) but not with Agatston CAC (adjusted beta co-efficient: –3.86 [95% CI, –24.37 to 16.66]; P = .712). CT emphysema was not associated with either Agatston CAC (adjusted beta co-efficient: –1.15 [95% CI, –3.03 to 0.73]; P = .230) or with Weston CAC (adjusted beta co-efficient: –0.003 [95% CI, –0.023 to 0.017]; P = .739) following adjustment for age, race, sex, smoking pack-years, and CT scanner type.
Visual CAC at 5-Year Follow-up
To test repeatability, the accuracy of Weston scores at the 5-year follow-up visit was also assessed. The Weston score was assessed in 1,869 participants (99.7%) and increased from a median of 3 (interquartile range, 0-6) at baseline to 4 (interquartile range, 1-7) at follow-up. The accuracy of Weston scores ≥ 7 for prevalent CAD at follow-up was 0.73 (95% CI, 0.69-0.77; P < .001), and for prevalent CVD, accuracy was 0.69 (95% CI, 0.65-0.72; P < .001).
Discussion
In a cohort of current and former smokers, with and without COPD, the present study found that CAC predicts incident cardiac events and also that a simple visual method of estimating CAC performs well in predicting prevalent CAD and incident cardiovascular disease. The visual score was equally accurate as the Agatston score for prevalent CAD and performed better than the Agatston score in predicting incident cardiac events. With COPD increasingly recognized as a cardiovascular risk factor, the early recognition of CAC is especially important for both the diagnosis of cardiovascular disease and for prognostication. Agatston CAC scores rely on relatively complex methods, and the Weston CAC score can provide equivalent prognostic information.
The utility of CAC in diagnosing cardiovascular disease and predicting incident disease has been extensively debated. Although early studies showed that CAC provides additional information over risk scores such as the Framingham risk score, recent studies have struggled to identify a distinct threshold for CAC as measured traditionally by using the Agatston scores. Whether any score > 0 implies presence of occult CAD is not clear. In addition, Agatston CAC scores have not been validated in COPD. One small case-control study of 162 subjects found that patients with COPD experienced greater coronary events despite no difference in CAC, suggesting that the excess risk could not be explained by CAC.15 The Multi-Ethnic Study of Atherosclerosis (MESA) lung study, which excluded patients with known cardiac disease, found that airflow obstruction was associated with subclinical atherosclerosis in the carotid and peripheral circulation but not when assessed according to Agatston CAC.13 Rasmussen et al24 found a relationship between COPD and CAC but no dose-response relationship between COPD severity and CAC. In contrast, we found that Agatston CAC was associated with lower FEV1. Our findings are in line with two studies from South Korea that found an inverse relationship between FEV1 and Agatston CAC14, 25 and support the results of multiple epidemiologic studies showing an association between lower FEV1 and CAD.3 We included patients across the spectrum of COPD severity as well as those with a high burden of cardiac disease. Results from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study suggest that the presence of CAC in COPD is associated with greater dyspnea, reduced exercise tolerance, and increased all-cause mortality; however, this study did not examine cardiovascular events.26 We extend the literature by demonstrating that Agatston CAC in COPD is associated with incident cardiovascular events.
Similarly, using CAC measured on an ordinal visual scale, O’Hare et al27 found that visual CAC > 4 in patients with COPD is associated with emphysema severity, myocardial infarction, and all-cause mortality. We also found that visually scored CAC was associated with airflow obstruction as well as with incident cardiovascular events. The visual score performed better than the Agatston score in predicting incident cardiovascular disease over 5 years, and we contend that the visual score is simpler and easier to adapt in daily clinical practice. The reasons for this difference in cardiac risk prediction are unclear. The Agatston score is a combination of plaque volume as well as density, and it is weighted more toward density.28 Calcification volume is likely a stronger predictor of cardiovascular disease, and hence the visual method might improve prediction by relying more on the size of the lesions detected than on the density. In addition, participants with early disease may have small or trace levels of calcification that may be < 3 contiguous voxels, or slightly less than the 130 HU threshold, and these lesions may end up being classified as undetectable. The original Agatston protocol usually has 3 mm collimation because the validation came from early CT studies that used the Imatron electron beam scanner, which had a minimum collimation of 3 mm. The current study protocol used submillimeter z-axis collimation and slice thickness. With the high rate of use of clinical CT scans of the lung for other indications, and an expected increase in the number of these scans for lung cancer screening in patients who share the same risk factors for COPD, the visual score can be easily adapted into clinical practice.29, 30
The present study has a number of limitations. The CAC scores were estimated by experienced radiologists, and hence these findings may not be generalizable. However, the visual scoring system is very simple and has excellent intra-observer and interobserver agreement, and with increasing recognition of the clinical importance of coronary calcification, many radiologists already report the presence of calcification. Although other visual scoring methods exist,29, 30 we chose the Weston score as representative of easy to use scoring methods. The CT scans were not electrocardiographically gated. However, recent studies have shown a strong correlation between gated and nongated scans, and CAC measured on nongated scans has been shown to be independently associated with clinical outcomes.11, 31, 32 It is possible that some events were not captured on follow-up, but this bias would likely affect both scores equally because the scores were estimated in the same participants. The study also has a number of strengths. Our analyses included participants from the COPDGene study, a well-characterized cohort with participants with all stages of COPD severity, included a high percentage of African-American subjects, had rigorous CT and spirometry quality control, and included participants with a high burden of cardiovascular disease.
Conclusions
With increasing recognition of cardiovascular disease as a major comorbidity in COPD, the use of a simple visual scale to identify and prognosticate patients adds to the clinical evaluation of these patients. There is considerable merit in using readily available clinical CT scans to screen for cardiovascular disease in this high-risk COPD population.
Acknowledgments
Author contributions: S. P. B. is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. S. P. B. and H. N. were responsible for study design. E. A. K., J. D. N., M. J. B., C. A. D., F. L. J., A. Y., C. F., J. H. T., and H. N. performed CAC measurements. S. P. B. conducted the statistical analyses. S. P. B., M. T. D., and H.N. wrote the manuscript. All authors were responsible for acquisition of data, data interpretation, and critical review of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. P. B. reports grants from the National Institutes of Health (NIH). M. J. B. reports grants from GE. M. T. D. reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study; grants from the Department of Defense; and personal fees and other from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Boston Scientific, other from Novartis, Yungjin, PneumRx/BTG, and Pulmonx, and personal fees from Genentech, outside the submitted work. J. D. N. reports grants from the NIH during the conduct of the study; and grants from the NIH, grants from Siemens HealthCare, and personal fees from VIDA Diagnostics Inc, outside the submitted work. None declared (E. A. K., J. E. H., C. A. D., C. H. M., S. B., F. L. J., A. Y., C. F., H. N.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was supported by the Genetic Epidemiology of COPD (COPDGene) study [National Institutes of Health grants U01 HL089897 and U01 HL089856 and K23HL133438 (S. P. B.)]. The COPDGene project is also supported by the COPD Foundation through contributions made to an industry advisory board composed of AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline. The coronary calcification data are supported by the Tobacco-Related Disease Research Program [award no. 20XT-0014].
Some of the findings of this study were presented at an oral presentation at the European Respiratory Society (ERS) Conference, September 3-7, 2016, London, England.
References
- 1.Bhatt S.P., Dransfield M.T. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res. 2013;162(4):237–251. doi: 10.1016/j.trsl.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Sin D.D., Man S.F. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2(1):8–11. doi: 10.1513/pats.200404-032MS. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Thomas J., Sadatsafavi M., FitzGerald J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 4.Portegies M.L., Lahousse L., Joos G.F. Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam Study. Am J Respir Crit Care Med. 2016;193(3):251–258. doi: 10.1164/rccm.201505-0962OC. [DOI] [PubMed] [Google Scholar]
- 5.Castagna O., Boussuges A., Nussbaum E., Marqueste L., Brisswalter J. Peripheral arterial disease: an underestimated aetiology of exercise intolerance in chronic obstructive pulmonary disease patients. Eur J Cardiovasc Prev Rehabil. 2008;15(3):270–277. doi: 10.1097/HJR.0b013e3282f009a9. [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino R.B., Sr., Grundy S., Sullivan L.M., Wilson P. CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 7.Taylor A.J., Feuerstein I., Wong H., Barko W., Brazaitis M., O'Malley P.G. Do conventional risk factors predict subclinical coronary artery disease? Results from the Prospective Army Coronary Calcium Project. Am Heart J. 2001;141(3):463–468. doi: 10.1067/mhj.2001.113069. [DOI] [PubMed] [Google Scholar]
- 8.Yeboah J., McClelland R.L., Polonsky T.S. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner D.J., Hall E.J. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 10.National Lung Screening Trial Research Team. Church T.R., Black W.C. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budoff M.J., Nasir K., Kinney G.L. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5(2):113–118. doi: 10.1016/j.jcct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts JR Jr, Dauer D.J., Fineberg N.S., Budoff M., Nath H. Correlation between measured and visual scoring of coronary artery calcification. Int J Cardiovasc Cerebrovasc Dis. 2014;2:11–17. [Google Scholar]
- 13.Barr R.G., Ahmed F.S., Carr J.J. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39(4):846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park H.Y., Lim S.Y., Hwang J.H. Lung function, coronary artery calcification, and metabolic syndrome in 4905 Korean males. Respir Med. 2010;104(9):1326–1335. doi: 10.1016/j.rmed.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Gaisl T., Schlatzer C., Schwarz E.I. Coronary artery calcification, epicardial fat burden, and cardiovascular events in chronic obstructive pulmonary disease. PLoS One. 2015;10(5):e0126613. doi: 10.1371/journal.pone.0126613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaha M.J., Budoff M.J., Tota-Maharaj R. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407–1416. doi: 10.1016/j.jcmg.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirsch J., Buitrago I., Mohammed T.L., Gao T., Asher C.R., Novaro G.M. Detection of coronary calcium during standard chest computed tomography correlates with multi-detector computed tomography coronary artery calcium score. Int J Cardiovasc Imaging. 2012;28(5):1249–1256. doi: 10.1007/s10554-011-9928-9. [DOI] [PubMed] [Google Scholar]
- 18.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vestbo J., Hurd S.S., Agusti A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 20.Wan E.S., Castaldi P.J., Cho M.H. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt S.P., Terry N.L., Nath H. Association between expiratory central airway collapse and respiratory outcomes among smokers. JAMA. 2016;315(5):498–505. doi: 10.1001/jama.2015.19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 23.Ho J.S., Fitzgerald S.J., Stolfus L.L. Relation of a coronary artery calcium score higher than 400 to coronary stenoses detected using multidetector computed tomography and to traditional cardiovascular risk factors. Am J Cardiol. 2008;101(10):1444–1447. doi: 10.1016/j.amjcard.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen T., Kober L., Pedersen J.H. Relationship between chronic obstructive pulmonary disease and subclinical coronary artery disease in long-term smokers. Eur Heart J Cardiovasc Imaging. 2013;14(12):1159–1166. doi: 10.1093/ehjci/jet057. [DOI] [PubMed] [Google Scholar]
- 25.Chae E.J., Seo J.B., Oh Y.M., Lee J.S., Jung Y., Lee S.D. Severity of systemic calcified atherosclerosis is associated with airflow limitation and emphysema. J Comput Assist Tomogr. 2013;37(5):743–749. doi: 10.1097/RCT.0b013e318299f9e7. [DOI] [PubMed] [Google Scholar]
- 26.Williams M.C., Murchison J.T., Edwards L.D. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax. 2014;69(8):718–723. doi: 10.1136/thoraxjnl-2012-203151. [DOI] [PubMed] [Google Scholar]
- 27.O'Hare P.E., Ayres J.F., O'Rourke R.L. Coronary artery calcification on computed tomography correlates with mortality in chronic obstructive pulmonary disease. J Comput Assist Tomogr. 2014;38(5):753–759. doi: 10.1097/RCT.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 28.Criqui M.H., Denenberg J.O., Ix J.H. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311(3):271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shemesh J., Henschke C.I., Shaham D. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257(2):541–548. doi: 10.1148/radiol.10100383. [DOI] [PubMed] [Google Scholar]
- 30.Chiles C., Duan F., Gladish G.W. Association of coronary artery calcification and mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276(1):82–90. doi: 10.1148/radiol.15142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutt A., Duhamel A., Deken V. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521–1528. doi: 10.1007/s00330-015-3978-7. [DOI] [PubMed] [Google Scholar]
- 32.Hughes-Austin J.M., Dominguez A., III, Allison M.A. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152–159. doi: 10.1016/j.jcmg.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]