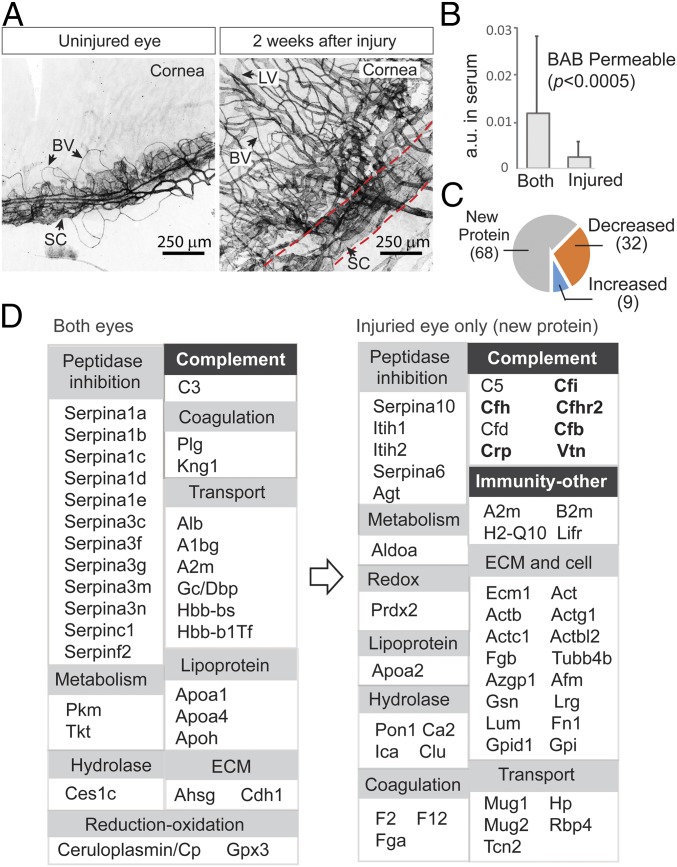
Fig. 3.
Changes in aqueous barrier permeability following neovascularization and wound healing. (A) In the eye model, one of the two eyes was first injured with a laser and then allowed to recover for 2 wk. Whole-mount immunofluorescence staining of limbal vascular plexus (between dotted lines) using anti-CD31 showed destruction of Schlemm’s canal (SC) and excessive neovascular growth of both blood vessels (BVs) and lymphatic vessels (LVs) in the recovering eye. (B) The abundance of individual serum proteins was measured by label-free quantification (y-axis). Those proteins with their 15N-labeled species detected in aqueous were considered BAB- permeable. These proteins were divided into two groups: those detected in both eyes and those detected in only the injured eye. New proteins that crossed the BAB due to injury are those with lower serum abundance compared with proteins that were permitted to enter both eyes, including the normal eye. (C) When the amounts of those proteins appearing in both injured and uninjured eyes were compared (at 5 h; the colored pies), a greater number of proteins had decreased levels (32 proteins) than had increased levels (9 proteins). (D) Functional classification of proteins that crossed the uninjured barrier (Left) vs. proteins that entered only the injured eye (Right). Expansions of groups such as complement system and other immunities are evident (highlighted). Proteins known to be associated with age-related macular degeneration are in bold type.