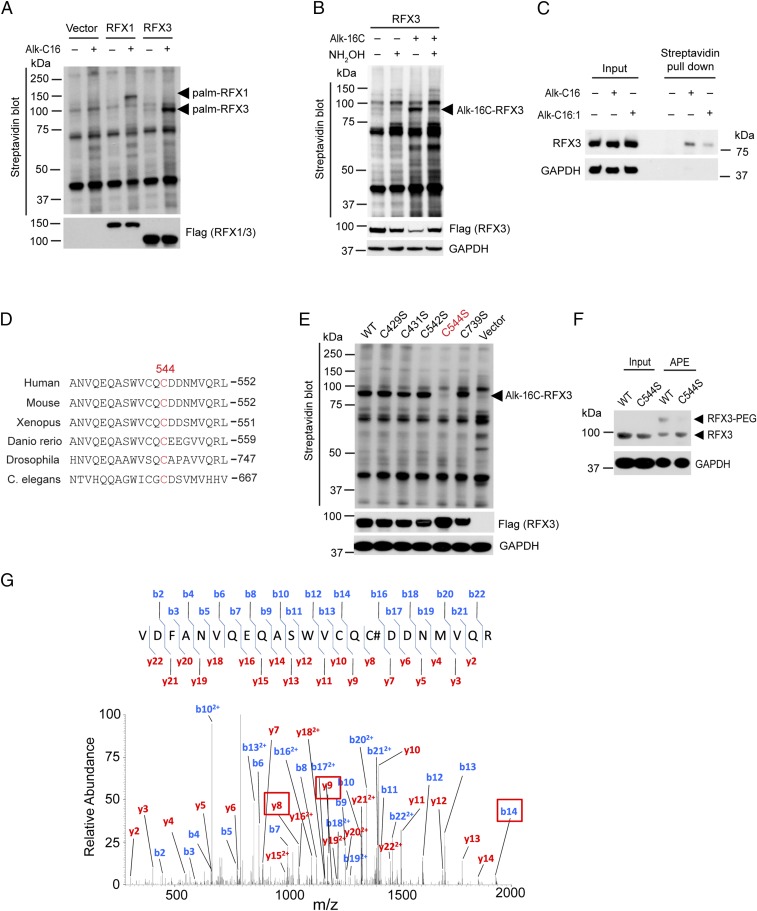
Fig. 1.
RFX3 is S-fatty acylated at an evolutionarily conserved cysteine residue. (A) Biochemical validation of RFX1 and RFX3 fatty acylation by metabolic labeling using chemical reporters of fatty acylation and streptavidin blot. (B) Treatment with hydroxylamine significantly decreased RFX3 fatty acylation. (C) Streptavidin pull-down and Western blotting confirmed that endogenous RFX3 is fatty acylated in human pancreatic β-cells. (D) Alignment of dimerization domain sequences of RFX3 family proteins by ClustalW, including human, mouse, Xenopus, Danio rerio, Drosophila, and C. elegans. (E) Mutation of cysteine 544 to serine completely abolished fatty acylation of RFX3. (F) APE assay confirmed S-fatty acylation of RFX3. (G) MS/MS spectral of RFX3 modified peptide showing S-fatty acylation on Cys544. #, IAA modification.