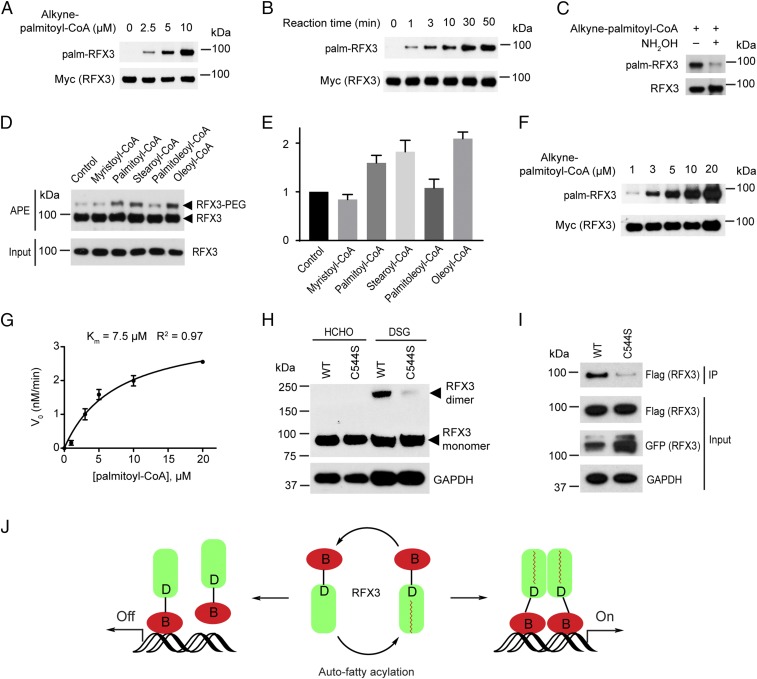
Fig. 3.
Auto-fatty acylation of RFX3 is required for its dimerization. (A) RFX3 is fatty acylated in a fatty acyl-CoA concentration-dependent manner in vitro. (B) RFX3 is fatty acylated in a time-dependent manner in vitro. (C) Hydroxylamine treatment substantially decreased in vitro fatty acylated RFX3. (D) Fatty acyl-CoA preferences of RFX3 in vitro by APE assay. (E) Quantification of in vitro fatty acylated RFX3 normalized by RFX3 protein level. (F) Representative streptavidin blot for measuring Km of in vitro fatty acylation of RFX3. (G) Plot of Km of in vitro fatty acylation of RFX3. Values represent the average ± SEM of three experiments. (H) Protein cross-linking results show stronger homodimerization of WT RFX3 than that of the fatty acylation-deficient C544S mutant. (I) Co-IP experiment shows the dimerization level decreased in the fatty acylation-deficient C544S mutant. (J) Cartoon showing fatty acylation of RFX3 regulates its dimerization and transcriptional activities.