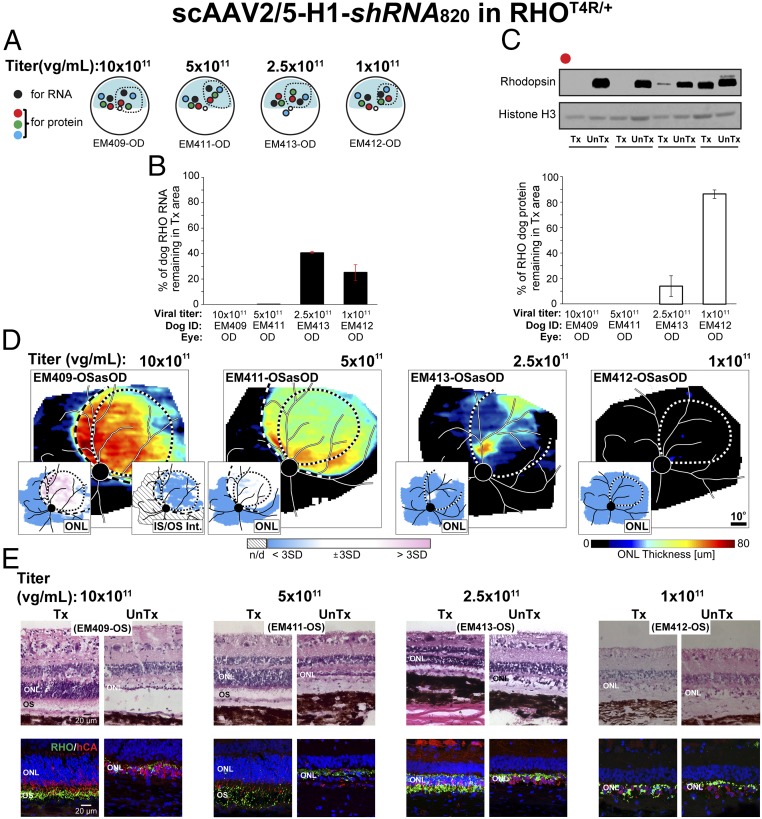
Fig. 3.
Suppression of rhodopsin with shRNA820 in RHO-mutant retinas. (A) Schematic representation of the fundus of four RHO-mutant dog eyes injected with scAAV2/5-H1-shRNA820 at 1 × 1011 to 10 × 1011 vg/mL titers showing the location of neuroretinal punches used for quantification of rhodopsin (RNA and protein) expression at 8–10 wk postinjection. Dashed lines indicate bleb boundaries; the blue area indicates the tapetal region. (B) Quantification of the levels of endogenous canine RHO RNA remaining in the treated retinal area as a percentage of levels measured in the untreated area of eyes injected with different titers. (C) Representative immunoblot and quantification of the levels of endogenous canine RHO protein remaining in the treated retinal area as a percentage of levels measured in the untreated area of eyes injected with 1 × 1011 to 50 × 1011 vg/mL titers. (D) ONL thickness topography 2 wk post light exposure (8–10 wk postinjection) in four RHO-mutant dog eyes treated with 1× 1011 to 10 × 1011 vg/mL titers. Dotted lines indicate bleb boundaries; dashed lines indicate ONL rescue boundaries. (Insets) Maps of significance showing retinal regions with ONL thickness (Left) and IS/OS intensity (Right) values compared point by point to the 99th percentile CIs of uninjected controls. (E) Microphotographs of H&E-stained (Upper) and rhodopsin (RHO, green)/human cone arrestin (hCA, red) coimmunolabeled (Lower) retinal cryosections showing morphology of the ONL and outer segment (OS) 2 wk post light exposure (8–10 wk postinjection) in areas treated with 1× 1011 to 10 × 1011 vg/mL titer range (Tx) and untreated areas (UnTx) of the eyes shown in D.