Abstract
Context:Nitraria retusa (Forssk.) Asch. (Nitrariaceae) is a medicinal plant which produces edible fruits whose antioxidant activity has been demonstrated.
Objective: The current study elucidates the potential protective effect of N. retusa fruit aqueous extract against nephrotoxicity induced by penconazole, a triazole fungicide, in the kidney of adult rats.
Materials and methods: Adult Wistar rats were exposed either to penconazole (67 mg/kg body weight), or to N. retusa extract (300 mg/kg body weight) or to their combination. Penconazole was administered by intra-peritoneal injection every 2 days from day 7 until day 15, the sacrifice day, while N. retusa extract was administered daily by gavage during 15 days. Oxidative stress parameters, kidney biomarkers and histopathological examination were determined.
Results:Nitraria retusa extract administration to penconazole treated rats decreased kidney levels of malondialdehyde (−10%), hydrogen peroxide (−12%), protein carbonyls (PCOs, −11%) and advanced oxidation protein products (AOPP, −16%); antioxidant enzyme activities: catalase (−13%), superoxide dismutase (−8%) and glutathione peroxidase (GPx, −14%), and the levels of non-enzymatic antioxidants: non-protein thiols (−9%), glutathione (−7%) and metallothionein (−12%). Furthermore, this plant extract prevented kidney biomarker changes by reducing plasma levels of creatinine, urea, uric acid and LDH and increasing those of ALP and GGT. Histopathological alterations induced by penconazole (glomeruli fragmentation, Bowman’s space enlargement, tubular epithelial cells necrosis and infiltration of inflammatory leucocytes) were attenuated following N. retusa administration.
Discussion and conclusion: Our results indicated that N. retusa fruit extract had protective effects against penconazole-induced kidney injury, which could be attributed to its phenolic compounds.
Keywords: Triazole fungicides, nephrotoxicity, nitrariaceae, polyphenols
Introduction
During the last decades, the use of pesticides has increased dramatically, worldwide, in an effort to increase food production and control vector-borne diseases. Nevertheless, these agrochemicals have been claimed to be harmful to the environment and to the human health (Hou & Wu 2010). Indeed, pesticides are released in large amounts in the environment, leading consequently to contamination of water, soil and air. Their residues have also been detected in food products prepared for human consumption (Cabras & Angioni 2000). The World Health Organization (WHO) estimates that there are 3 million cases of pesticides poisoning in each year and up to 220,000 deaths. Moreover, epidemiological studies have stated a causal connection between human exposure to pesticides and a number of health outcomes including endocrine disrupting effects (Goetz et al. 2007), mutagenicity (Bhinder & Chaudhry 2013), neurotoxicity (Galal et al. 2014), carcinogenicity (Greim et al. 2015), hepatotoxicity (Ogutcu et al. 2008), and nephrotoxicity (Hou et al. 2014).
Among pesticides, penconazole [1-(2-(2,4-dichlorophenyl)-pentyl)-1H-1,2,4-triazole], a systemic triazole fungicide with preventive and curative properties, is widely and effectively used throughout the world for the prevention and control of powdery mildew disease in vineyards and other crops. This fungicide belongs to conazoles, a class of azole-based fungicides widely used in agriculture as well as in human and veterinary medicine applications for the treatment of local and systemic fungal infections. It acts by inhibiting a specific cytochrome P450, lanosterol-14α-demethylase (CYP51), which is required for ergosterol biosynthesis in order to maintain the proper membrane fluidity and permeability in fungi (Zarn et al. 2003). Penconazole is classified by WHO as Class U to present, unlikely, an acute hazard in normal use. In a previous study, this fungicide has been shown to be toxic to the reproductive system of male rats (El-Sharkawy & El-Nisr 2013). In addition, we have recently demonstrated that penconazole is a potent hepatotoxic and cardiotoxic (Chaâbane et al. 2015, 2016) fungicide in rats. Furthermore, toxicological studies have shown an extensive oral absorption, a wide distribution in the body and an excretion of this fungicide predominately via urine, where numerous metabolites have been identified.
Since the kidney is the major organ for xenobiotics release, renal cells are thus exposed to high doses of metabolites, causing nephrotoxicity. So, kidney is considered as a sensitive organ against pesticides-induced toxicity and damage (Kalendar et al. 2007). Indeed, various pesticides have been demonstrated to cause renal damage (Ahmed et al. 2010; Shah & Iqbal 2010; Ben Amara et al. 2013). Oxidative stress and reactive oxygen species (ROS) production have been reported as the main mechanism of the nephrotoxicity (Shah & Iqbal 2010). ROS, when produced in excess, could damage critical molecules such as lipids, proteins and nucleic acid bases, thereby resulting in cell cycle arrest and apoptosis (Perry et al. 2001; Barnham et al. 2004; Ting et al. 2010).
To counteract oxidative stress, endogenous and exogenous antioxidants play a crucial role to remove ROS. They act as free radical scavengers preventing cells and tissue damage. Exogenous antioxidants obtained from natural sources are considered to be relatively safe and without undesirable side effects (Xavier et al. 2004). Medicinal plants and herbs constitute a promising source of natural antioxidants. Many plant extracts are endowed with a variety of free radical scavenging molecules. Among them, phenolic compounds are well-known for their antioxidative activity, attributed mainly to their redox properties allowing them to act as the reducing agents, the hydrogen donors, the singlet oxygen quenchers as well as the metal chelators (Rice-Evans et al. 1995).
Nitraria retusa (Forssk.) Asch. (Nitrariaceae), a native perennial species, is a salt-tolerant and drought-resistant shrub, which produces fleshy red fruits eaten by humans (Le Floc’h 1983). A tasty refreshing juice may be extracted from the mature fruits. This plant is distributed in North Africa including Tunisia. Commonly known as ‘Ghardaq’, N. retusa is used in traditional medicine. The dry leaves serve as supplement to tea and used to make cataplasms. The ashes of this plant are applied on infected wounds in order to remove liquids (blood, lymph) and to facilitate the healing process (Shaltout et al. 2003). We have previously shown that the aqueous extract obtained from N. retusa fruit can serve as a source of bioactive compounds such as polyphenols and flavonoids (Chaâbane et al. 2014). We have also demonstrated that this extract exerts in vitro (Chaâbane et al. 2014) as well as in vivo (Chaâbane et al. 2015), a significant antioxidant activity. Nevertheless, the phenolic composition of N. retusa fruit has not been previously described.
To the best of our knowledge, no studies have examined the potential penconazole-induced nephrotoxicity. Therefore, the present study was conducted to assess some biochemical parameters, antioxidant status and histopathological alterations in the kidney of penconazole-exposed rats. We tried also to identify, for the first time, the phenolic compounds present in the aqueous extract of N. retusa fruit and to determine whether the treatment with this extract could alleviate penconazole-induced nephrotoxicity.
Materials and methods
Chemicals
Penconazole is a triazole fungicide (C13H15Cl2N3). The commercial formulation studied in the present work was Topas®, which contained 100 g/L of penconazole, the active ingredient, and was produced by Syngenta Company (Bâle, Suisse). Glutathione (GSH), 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), thiobarbituric acid, 1,1,3,3-tetrathoxypropane (TEP), 2,4-dinitrophenylhydrazine (DNPH) and nitro blue tetrazolium (NBT) were purchased from Sigma Chemical Co. (St. Louis, MO). The other ones of analytical grade were purchased from standard commercial suppliers.
Plant material
Fresh ripe fruits of N. retusa were collected from Kerkennah Island (Sfax, Tunisia). The plant was identified in botanic laboratory (Faculty of Sciences, University of Sfax, Tunisia), according to the Flora of Tunisia (Pottier-Alapetite 1979).
Mineral composition of N. retusa fruit
The content of magnesium (Mg2+), sodium (Na+), calcium (Ca2+), zinc (Zn2+), iron (Fe2+) and copper (Cu2+) in N. retusa fruit was determined by atomic absorption spectrometry (Sherwood 410) after digestion with concentrated nitric acid (AOAC 2000). Briefly, after being separated from the seeds, the fruit pulps of N. retusa were homogenized in a domestic blender (Ufesa Supplier, Spain). An amount of 1 g of the homogenate was dried at 105 °C for 24 h and subsequently incinerated in a muffle furnace at 550 °C for 4 h. The residue of incineration was cooled, dissolved in 1 mL of concentrated nitric acid and allowed to stand for 30 min. After that, the sample was diluted with 10 mL of deionized water and filtered with Whatman No.1 filter paper. Then, the solution was transferred to a volumetric flask and diluted to a final volume of 25 mL with deionized water. The resulting extract was used for mineral content determination. Results were expressed as mg/100 g N. retusa fresh weight (NRFW).
Preparation of N. retusa fruit aqueous extract
An aqueous extract of N. retusa fruit was prepared following the procedure of Benvenuti et al. (2004), with slight modifications. Briefly, 200 g of the previously prepared fruit homogenate were extracted with 200 mL distilled water under continuous stirring for 60 min. The extract was filtered under vacuum through Whatman No.1 filter paper on a Buchner funnel, and the residue was extracted again by the same way. The obtained filtrates were combined, lyophilized and kept in the dark at 4 °C until use.
High-pressure liquid chromatography–high resolution mass spectrometry (HPLC-HRMS) analysis
The phenolic compounds present in the aqueous extract of N. retusa fruit were analyzed using ultra high-pressure liquid chromatography (UPLC 1290 Infinity) coupled with high resolution–mass spectrometry (HR-MS Q-TOF UHD 6538) from Agilent Technologies. In brief, chromatographic separation was carried out using a Thermo Hypersyl Gold column (4.6 × 150 × 5 μm) at a flow rate of 0.7 mL/min. The separation gradient was generated using 0.1% formic acid in water (solvent A), and acetonitrile (solvent B). The gradient was defined as follows: from 0 to 20 min, 6% B; from 20 to 35 min, 18% B; from 35 to 45 min, 28% B; from 45 to 48 min, 60% B; from 48 to 55 min, 90% B. Cleaning of the column was achieved with 90% B for 5 min. The mass spectra were acquired using a dual ESI ionization in positive-ion mode. The nebulization gas and the ion spray voltage were adjusted to 30 psi and 3.5 kV, respectively. The temperature of the ion source was fixed to 300 °C. All data were acquired and processed using Mass Hunter B.05.01 software. The identification was performed according to a home-generated database containing 500 compounds and a mixture of standards containing 30 phenolic compounds, as described by Koubaa et al. (2015).
Animals and treatment
A total of 24 male Wistar rats (251 ± 3 g) were purchased from the Central Pharmacy (SIPHAT, Tunisia). The rats were housed under standard laboratory conditions of temperature (22 ± 2 °C), relative humidity (40%) and 12 h light/dark cycles. They were fed with standard pellet diet (SNA, Sfax, Tunisia) and given drinking water ad libitum. Approval for rat experiments was obtained from the ethical Committee at Sciences Faculty of Sfax with ethics approval number 1204 and all the experimental procedures were in accordance with the International Guidelines for Animal Care (Council of European Communities 1986). The animals were randomly divided into four groups of six each as follows:
Group 1 (Controls): rats received distilled water.
Group 2 (PEN): no treatment was performed during the first 6 days, then rats received intraperitoneally 67 mg/kg body weight (1/30 LD50) of penconazole every 2 days from day 7 until day 15, the sacrifice day.
Group 3 (NRE + PEN): received daily by gavage during the first six days 300 mg/kg body weight of lyophilized N. retusa extract (corresponding, for each rat weighing a mean value of 251 g, to 75 mg of lyophilized N. retusa extract dissolved in 1 mL of distilled water). From day 7 until day 15, rats received aqueous extract of N. retusa fruit (300 mg/kg body weight) daily by gavage and penconazole (67 mg/kg body weight) every 2 days by intraperitoneal way.
Group 4 (NRE): serving as positive controls, received daily by gavage N. retusa aqueous extract (300 mg/kg body weight) for 15 days.
The dose of penconazole used in the present work was selected on the basis of the previous study carried out by El-Sharkawy and El-Nisr (2013). These authors have used two doses of penconazole, 50 mg/kg body weight and 100 mg/kg body weight (representing, respectively, 1/40 and 1/20 of LD50), which induce structural and functional testicular impairment in adult male rats. For our experiment, we have chosen an intermediate dose (67 mg/kg body weight), which represented 1/30 LD50 and caused toxicity without lethality. Indeed, a dose higher than 67 mg/kg body weight provoked haemorrhage and diarrhoea. Concerning N. retusa extract, a preliminary dose-response study showed that a dose higher than 300 mg/kg body weight was found to cause diarrhoea in exposed rats, which could be due to the presence of fibres in N. retusa fruits as reported by Hegazy et al. (2013).
Twenty four hours before the sacrifice day, the rats were placed individually in metabolic cages for urine collection. After recording 24 h urine volumes, samples were centrifuged at 5000 g for 5 min to eliminate the probable presence of excrements. The supernatants were collected and then stored at −20 °C for further analysis. At the end of the experiment and after 15 days of treatment, the rats were sacrificed by cervical dislocation to avoid stress condition. Blood samples were collected into heparin-coated tubes and centrifuged at 2200 g for 10 min. Plasma samples were drawn and stored at −20 °C until analysis. Kidney tissues were dissected out, cleaned from adipose tissue and weighed. Some samples were rinsed, homogenized in Tris-HCl buffer (pH 7.4) and centrifuged. The resulting supernatants were collected and kept at −80 °C until biochemical analysis. Other samples were fixed in 10% buffered formalin solution and embedded in paraffin for histological examination. For the biochemical and the histological experiments, samples (kidney tissue, plasma, urine) were taken from six rats in each group.
Biochemical assays
Protein quantification in kidneys
Kidney protein content was determined by Lowry et al. (1951) method using bovine serum albumin as standard.
Estimation of urea, uric acid, creatinine and creatinine clearance
The levels of urea, uric acid and creatinine in plasma and urine were assayed spectrophotometrically using commercially available diagnostic kits (Biomaghreb, Tunisia, Ref. 20143, 20095, 20151, respectively). Creatinine clearance was calculated using UV/P equation (Charrel 1991), where U is the urinary creatinine level, V the urinary volume collected within 24 h and P the plasma creatinine level.
Kidney and plasma lactate dehydrogenase activities
Lactate dehydrogenase (LDH) activities in the kidney and plasma were measured using a commercially available diagnostic kit (Biomaghreb, Tunisia, Ref. 200125).
Plasma alkaline phosphatase and gamma glutamyltranspeptidase activities
Plasma alkaline phosphatase (ALP) and gamma glutamyltranspeptidase (GGT) activities were determined using diagnostic kits (Biomaghreb, Tunisia, Ref. 20015 and 20022, respectively).
Evaluation of kidney lipid peroxidation
The concentrations of malondialdehyde (MDA), an index of lipid peroxidation, in kidney tissue were determined according to the method by Draper and Hadley (1990). The MDA values were calculated using TEP as standard and expressed as nmol of MDA/g tissue.
Estimation of hydrogen peroxide generation
Hydrogen peroxide (H2O2) generation in kidney tissue was assessed by Ou and Wolff method (Ou & Wolff 1996). The amount of H2O2 in the sample was determined using the extinction coefficient of 2.67 × 105 cm−1 M−1 and results were expressed as nmol/mg protein.
Determination of protein carbonyl levels in kidney
Protein carbonyl (PCO) content in kidney tissue was measured using the DNPH method by Reznick and Packer (1994). Results were expressed as nmol/mg protein.
Determination of advanced oxidation protein product levels in kidney
The kidney levels of advanced oxidation protein products (AOPP) were determined according to the method of Kayali et al. (2006), using the extinction coefficient of 261 cm−1 mM−1. Results were expressed as nmol/mg protein.
Determination of antioxidant enzyme activities
The catalase (CAT) activity was assayed as described by Aebi (1984) using H2O2 as substrate. The decrease in absorbance due to H2O2 degradation was recorded at 240 nm and CAT activity was calculated in terms of μmol H2O2 consumed/min/mg of protein.
The superoxide dismutase (SOD) activity was determined by monitoring the photochemical reduction of NBT according to the method of Beauchamp and Fridovich (1971). One unit of SOD activity corresponded to the amount of enzyme required to cause 50% inhibition of NBT reduction at 560 nm. SOD activity was expressed as units/mg protein.
The glutathione peroxidase (GPx) activity was estimated according to the method of Flohe and Gunzler (1984). The enzyme activity was expressed as nmol of GSH oxidized/min/mg protein.
Determination of non-protein thiol levels
Kidney non-protein thiol (NPSH) levels were determined by the method by Ellman (1959) and the results were expressed as μmol/g tissue.
Determination of GSH levels
The GSH content in kidney tissue was assayed according to the method by Ellman (1959) modified by Jollow et al. (1974). Results were expressed as μg/g tissue.
Determination of metallothionein content
The concentration of metallothionein (MT) in kidney tissue was estimated according to the method by Viarengo et al. (1997) modified by Petrovic et al. (2001). Results were expressed as μmol GSH/g tissue.
Histological studies
For histological examination, some kidneys were removed from control and treated rats. They were fixed in 10% buffered formalin solution and then embedded in paraffin. Sections of 5 μm thickness were placed on slides and stained with haematoxylin and eosin for histological evaluation under light microscopy. Six slides were prepared from each kidney tissue. All sections were evaluated for the degree of tubular and glomerular injury. Each kidney slide was examined and assigned for severity of changes using scores on a scale of none (−), mild (+), moderate (++) and severe (+++) damage.
Statistical analysis
The data were analyzed using the statistical package program Stat view 5 Software for Windows (SAS Institute, Berkley, CA). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Fisher protected least significant difference (PLSD) test as a post hoc test for comparison between groups. Student unpaired t test, used for comparison between two groups, was also required. All values were expressed as means ± SD. Differences were considered significant if p < 0.05.
Results
In vitro study
Mineral analysis of N. retusa fruit
The mineral content of N. retusa fruit was determined in the present study (Table 1). The most abundant macro-element was Na+ (410.82 mg/100 g NRFW) while Ca2+ and Mg2+ were found in smaller amounts than Na+ (101.82 and 54.69 mg/100 g NRFW, respectively). Regarding the microelements, the highest value was represented by Fe2+ (6.78 mg/100 g NRFW), followed by Zn2+ (0.48 mg/100 g NRFW) and Cu2+ (0.322 mg/100 g NRFW).
Table 1.
Mineral content of N. retusa fruit.
| Minerals | Concentration (mg/100 gN. retusa fresh weight) |
|---|---|
| Mg2+ | 54.69 |
| Na+ | 410.82 |
| Ca2+ | 101.82 |
| Zn2+ | 0.48 |
| Fe2+ | 6.78 |
| Cu2+ | 0.322 |
Mg2+: magnesium, Na+: sodium, Ca2+: calcium, Zn2+: zinc, Fe2+: iron, Cu2+: copper.
Polyphenols identification
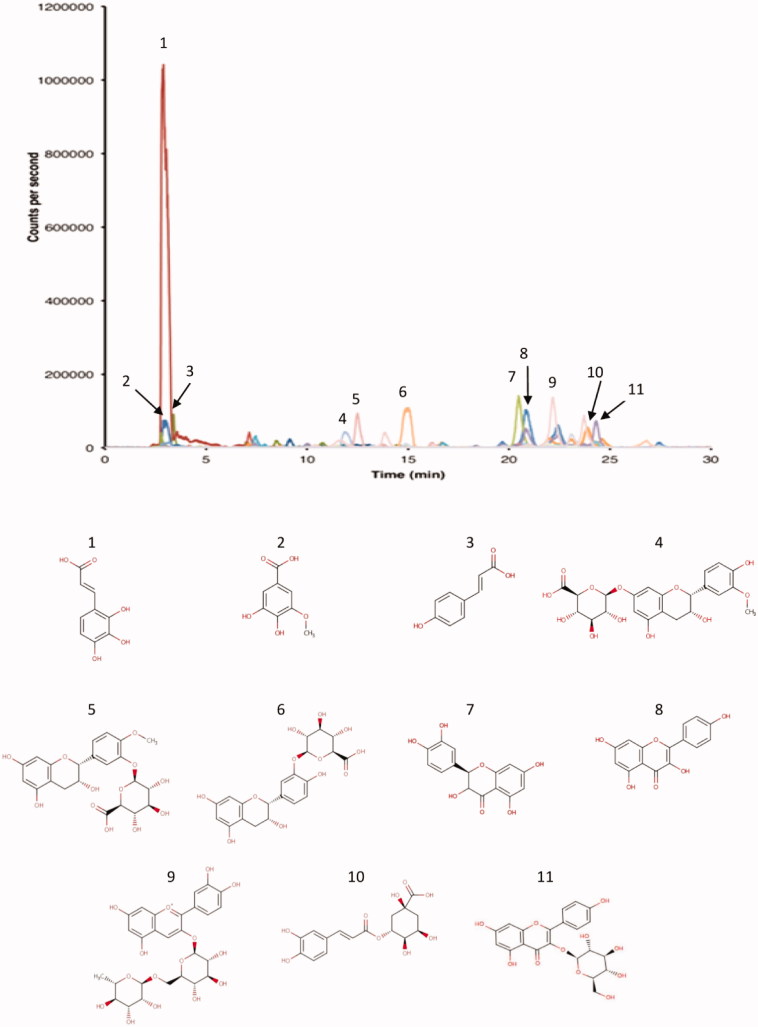
The phenolic compounds profile of N. retusa fruit aqueous extract is shown in Figure 1. Based on analysis by HPLC-HRMS, the major identified components were hydroxycaffeic acid (1), 3-O-methylgallic acid (2), p-coumaric acid (3), 3′-O-methyl-(-)-epicatechin 7-O-glucuronide (4), 4′-O-methyl-(-)-epicatechin 3′-O-glucuronide (5), epicatechin 3′-O-glucuronide (6), taxifoline (7), kaempferol (8), cyanidin 3-O-rutinoside (9), chlorogenic acid (10) and kaempferol 3-glucoside (11).
Figure 1.
HPLC–HRMS chromatogram representing the polyphenols extracted from the aqueous extract of N. retusa fruit and chemical structure of the major components identified [hydroxycaffeic acid (1), 3-O-methylgallic acid (2), p-coumaric acid (3), 3′-O-methyl-(-)-epicatechin 7-O-glucuronide (4), 4′-O-methyl-(-)-epicatechin 3′-O-glucuronide (5), epicatechin 3′-O-glucuronide (6), taxifoline (7), kaempferol (8), cyanidin 3-O-rutinoside (9), chlorogenic acid (10) and kaempferol 3-glucoside (11)]. The identification was performed according to a home-generated database containing 500 compounds and a mixture of standards containing 30 phenolic compounds.
In vivo study
Body, absolute and relative kidney weights. Food and water intakes
During the experimental period, there was no observed mortality in any experimental group. Moreover body, absolute and relative kidney weights as well as the daily food intake of treated animals were similar to those of controls. While a significant increase (+30%) in the daily water consumption was shown in penconazole-treated rats, as compared to the controls (Table 2).
Table 2.
Body, absolute and relative kidney weights. Food and water intake of control and treated rats with penconazole (PEN), N. retusa aqueous extract (NRE), or their combination (NRE + PEN).
| Parameters and treatments | Controls | PEN | NRE + PEN | NRE |
|---|---|---|---|---|
| Initial body weight (g) | 248.67 ± 8.48 | 249.67 ± 5.61 | 251.67 ± 4.27 | 254.17 ± 6.43 |
| Final body weight (g) | 261.00 ± 6.81 | 264.33 ± 5.20 | 262.00 ± 4.86 | 267.67 ± 9.18 |
| Absolute kidney weight (g) | 1.61 ± 0.15 | 1.68 ± 0.10 | 1.61 ± 0.06 | 1.76 ± 0.17 |
| Relative kidney weight (g/100g bw) | 0.62 ± 0.05 | 0.63 ± 0.03 | 0.61 ± 0.02 | 0.66 ± 0.05 |
| Food intake (g/day/rat) | 16.68 ± 1.59 | 16.08 ± 1.77 | 15.89 ± 1.55 | 16.39 ± 1.42 |
| Water intake (mL/day/rat) | 25.47 ± 2.45 | 33.16 ± 2.64*** | 26.07 ± 2.22### | 26.03 ± 1.64 |
Values are means ± SD for six rats in each group.
bw: body weight.
PEN group vs. control group:
p < 0.001.
NRE + PEN group vs PEN group:
p < 0.001.
Biomarkers of renal toxicity
Results showed that creatinine, urea and uric acid levels of penconazole-treated rats were higher in plasma (+20, +61 and +65%, respectively) and lower in urine (−71, −66 and −47%) than those of the controls (Table 3). These observations were associated with a significant elevation of 24 h urinary volume and a reduction in creatinine clearance (Table 3). Treatment of penconazole-treated rats with N. retusa extract improved the levels of renal biomarkers, compared to those of PEN group.
Table 3.
Urinary volume, creatinine clearance, plasma and urinary levels of creatinine, urea and uric acid, and BUN of control and treated rats with penconazole (PEN), N. retusa aqueous extract (NRE) or their combination (NRE + PEN).
| Parameters and treatments | Controls | PEN | NRE + PEN | NRE |
|---|---|---|---|---|
| Urinary volume (mL/24 h) | 5.33 ± 0.41 | 14.00 ± 1.87*** | 11.67 ± 0.41***## | 7.67 ± 1.08*** |
| Creatinine clearance (μL/min) | 536.75 ± 81.31 | 339.58 ± 39.50*** | 533.28 ± 88.10### | 640.26 ± 83.20* |
| Creatinine (μmol/L) | ||||
| Plasma | 52.40 ± 3.12 | 62.62 ± 6.12** | 54.67 ± 4.86# | 54.34 ± 0.96 |
| Urine | 7551.25 ± 573.05 | 2216.67 ± 440.08*** | 3580.63 ± 526.25***### | 6550.00 ± 39.53** |
| Urea (mmol/L) | ||||
| Plasma | 5.82 ± 0.39 | 9.35 ± 0.48*** | 7.38 ± 0.25***### | 6.49 ± 0.23** |
| Urine | 472.61 ± 66.84 | 161.22 ± 9.60*** | 234.16 ± 31.28***### | 359.57 ± 19.09** |
| Uric acid (μmol/L) | ||||
| Plasma | 73.13 ± 6.04 | 120.71 ± 9.60*** | 64.53 ± 5.30***# | 76.93 ± 3.70 |
| Urine | 247.50 ± 39.47 | 131.67 ± 14.72*** | 147.5 ± 7.58***# | 215.00 ± 30.17 |
| BUNa (mmol/L) | 2.72 ± 0.18 | 4.37 ± 0.22*** | 3.45 ± 0.12***### | 3.06 ± 0.11** |
Values are means ± SD for six rats in each group.
BUN: blood urea nitrogen.
BUN was calculated using the formula conversion:
BUN (mmol/L) = [urea(mmol/L)]/2.14.
PEN, NRE + PEN and NRE groups vs. control group:
p < 0.05.
p < 0.01.
p < 0.001.
NRE + PEN group vs PEN group:
p < 0.05.
p < 0.01.
p < 0.001.
Plasma ALP, GGT and LDH activities and kidney LDH activity
In penconazole-treated rats, plasma ALP and GGT activities decreased by 22 and 35%, respectively, when compared to controls (Table 4). In addition, LDH activity increased by 57% in the plasma while it decreased by 22% in the kidney of penconazole-treated rats, when compared to the controls (Table 4). Administration of N. retusa extract increased the activities of ALP and GGT by 8 and 28%, respectively, as compared to penconazole-treated rats. Moreover, in NRE + PEN group, LDH activity decreased by 30% in the plasma and increased by 13% in the kidney, as compared to PEN group (Table 4).
Table 4.
Plasma ALP, GGT and LDH activities and kidney LDH activity in control and treated rats with penconazole (PEN), N. retusa aqueous extract (NRE) or their combination (NRE + PEN).
| Parameters and treatments | Controls | PEN | NRE + PEN | NRE |
|---|---|---|---|---|
| Plasma | ||||
| ALP (IU/L) | 122.5 ± 13.65 | 95.42 ± 5.94** | 103.08 ± 5.60** | 112.67 ± 5.49 |
| GGT (IU/L) | 11.50 ± 1.75 | 7.44 ± 0.50*** | 9.52 ± 0.84*### | 12.30 ± 2.43 |
| LDH (IU/L) | 1162.33 ± 97.84 | 1824.17 ± 94.96*** | 1274.67 ± 89.08*### | 1312.00 ± 21.43** |
| Kidney | ||||
| LDH (IU/L) | 3.37 ± 0.29 | 2.63 ± 0.23*** | 2.97 ± 0.29*# | 3.27 ± 0.22 |
Values are means ± SD for six rats in each group.
ALP: alkaline phosphatase, GGT: gamma glutamyltranspeptidase, LDH: Lactate dehydrogenase.
PEN, NRE + PEN and NRE groups vs. control group:
p < 0.05.
p < 0.01.
p < 0.001.
NRE + PEN group vs PEN group:
p < 0.05.
p < 0.001.
Lipid peroxidation in the kidney
Our results revealed an increase of lipid peroxidation in the kidney of penconazole-treated rats as evidenced by the enhanced MDA level (+67%) when compared to controls. In NRE + PEN group, the MDA level was significantly reduced (−10%) compared to the PEN group (Table 5).
Table 5.
Kidney MDA, H2O2, PCO, AOPP, NPSH, GSH and MT levels in control and treated rats with penconazole (PEN), N. retusa aqueous extract (NRE) or their combination (NRE + PEN).
| Parameters and treatments | Controls | PEN | NRE + PEN | NRE |
|---|---|---|---|---|
| MDA (nmol/g tissue) | 142.75 ± 9.63 | 238.01 ± 18.62*** | 213.81 ± 15.14***# | 147.22 ± 16.49 |
| H2O2 (nmol/mg protein) | 0.042 ± 0.004 | 0.064 ± 0.005*** | 0.056 ± 0.005***# | 0.045 ± 0.004 |
| PCO (nmol/mg protein) | 0.59 ± 0.05 | 0.84 ± 0.05*** | 0.75 ± 0.06***# | 0.56 ± 0.05 |
| AOPP (nmol/mg protein) | 0.20 ± 0.02 | 0.37 ± 0.02*** | 0.31 ± 0.01***### | 0.21 ± 0.02 |
| NPSH (μmol/g tissue) | 2.40 ± 0.12 | 2.76 ± 0.05*** | 2.50 ± 0.04*### | 2.47 ± 0.07 |
| GSH (μg/g tissue) | 29.74 ± 1.45 | 34.05 ± 2.30** | 31.55 ± 1.08*# | 30.86 ± 0.71 |
| MT (μmol GSH/g tissue) | 0.49 ± 0.01 | 0.57 ± 0.06** | 0.50 ± 0.03# | 0.47 ± 0.04 |
Values are means ± SD for six rats in each group.
MDA: malondialdehyde, H2O2: hydrogen peroxide, PCO: protein carbonyls, AOPP: advanced oxidation protein products, NPSH: non-protein thiols, GSH: reduced glutathione MT: metallothionein.
PEN and NRE + PEN groups vs. control group:
p < 0.05.
p < 0.01.
p < 0.001.
NRE + PEN group vs PEN group:
p < 0.05.
p < 0.001.
Hydrogen peroxide generation in the kidney
The level of H2O2 generated in kidney was significantly increased (+52%) in the PEN group when compared to controls. Administration of N. retusa extract to penconazole-treated rats decreased significantly (−12%) H2O2 level as compared to the PEN group.
Protein oxidative markers in the kidney
In the PEN group, a significant increase of PCO and AOPP levels in the kidney tissue of adult rats (+42 and +85%, respectively) was observed when compared to the controls. Treatment with N. retusa extract resulted in a marked decrease of kidney PCO and AOPP levels (−11 and −16%, respectively) when compared to the Pen group (Table 5).
Non-enzymatic antioxidant status in the kidney
The levels of non-enzymatic antioxidants such as NPSH, GSH and MT were determined in the kidney of experimental animals. Results showed that penconazole treatment increased significantly the levels of NPSH (+15%), GSH (+16%) and MT (+16%), as compared to controls (Table 5). These modifications were alleviated following administration of N. retusa extract to penconazole treated rats as indicated by the significant decrease of NPSH (−9%), GSH (−7%) and MT (−12%) levels compared to those of PEN group.
Enzymatic antioxidant status in the kidney
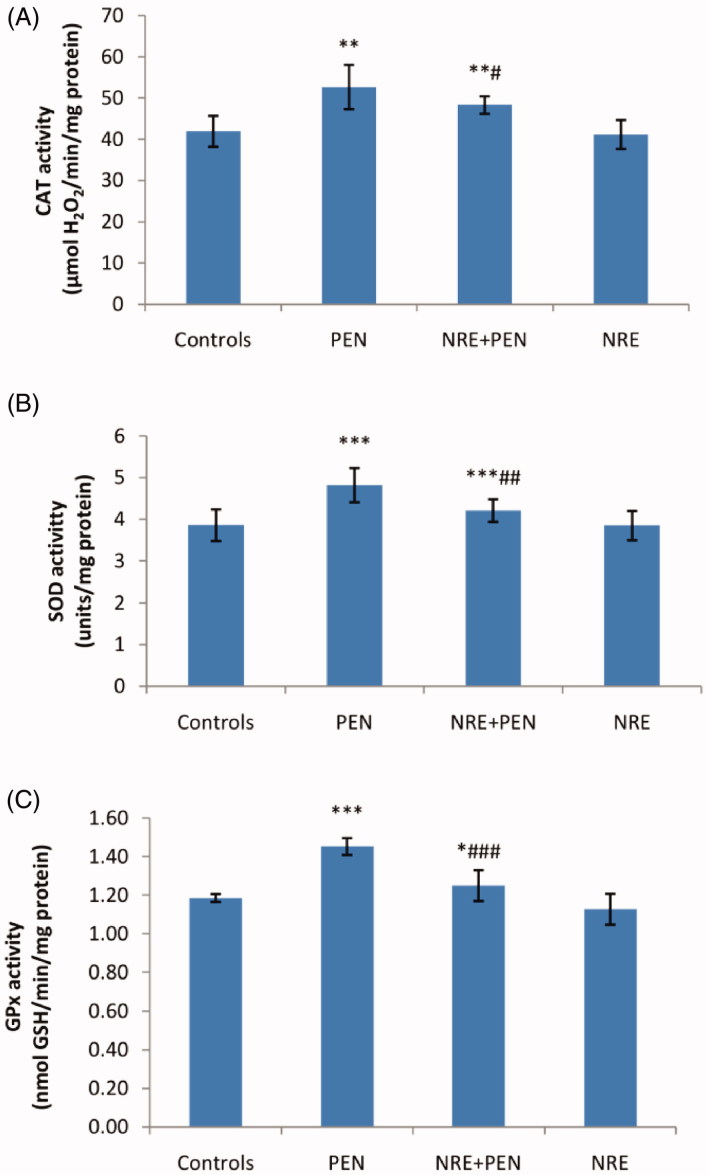
Penconazole treatment resulted in a significant increase of CAT (+26%), SOD (+25%) and GPx activities (+23%) when compared to controls. Treatment with N. retusa aqueous extract reduced significantly the activities of these enzymes by 8, 13 and 14%, respectively, compared to those of penconazole-treated group. Treatment of adult rats with N. retusa extract alone produced no changes in the activities of these enzymes, when compared to those of negative controls (Figure 2).
Figure 2.
Antioxidant enzyme activities (A) CAT, (B) SOD and (C) GPx in kidney of control and treated rats with penconazole (PEN), N. retusa aqueous extract along with penconazole (NRE + PEN) and N. retusa aqueous extract (NRE). Values are means ± SD for six rats in each group. PEN and NRE + PEN groups vs control group: *p < 0.05; **p < 0.01; ***p < 0.001. NRE + PEN group vs PEN group: #p < 0.05; ##p < 0.01; ###p < 0.001
Kidney histoarchitecture
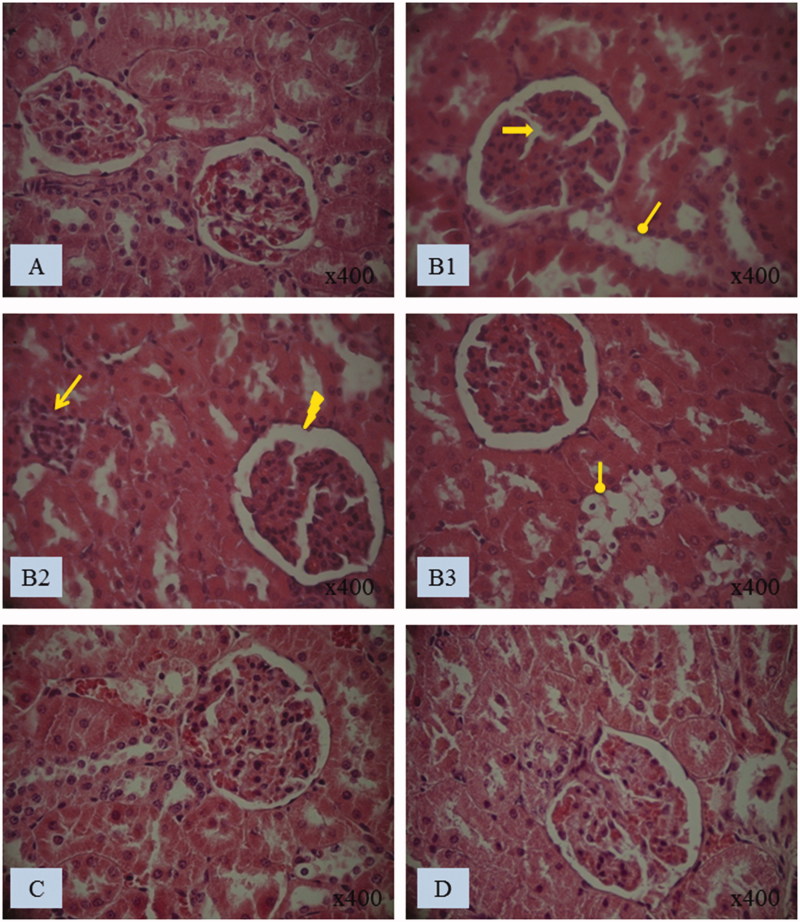
Histological studies showed that control rats presented a normal kidney histoarchitecture with normal tubules and intact glomeruli (Figure 3(A)). While kidney histology of the PEN group revealed several abnormalities, detected both in the tubules and in glomeruli, when compared to the controls. In fact, penconazole treatment caused necrosis of the epithelial cells lining the tubules (Figure 3(B1,B3)), in addition to the infiltration of inflammatory leucocytes (Figure 3(B2)). Glomeruli fragmentation and Bowman’s space enlargement were also observed in the kidney of penconazole-treated rats (Figure 3(B1,B2)). The co-administration of N. retusa extract to penconazole-treated rats reduced markedly the glomeruli fragmentation and the Bowman’s space enlargement when compared to the PEN group (Figure 3(C)). Furthermore, this extract attenuated the infiltration of inflammatory leucocytes and the damages to the epithelial cells lining the tubules induced by penconazole (Figure 3(C)). Microscopic examination of kidney sections of NRE group showed normal histological structure of renal tubules and glomeruli (Figure 3(D)). The histological changes in treated rats were graded and summarized in Table 6.
Figure 3.
Histological kidney sections of (A) control and (B1, B2 and B3) treated rats with penconazole, (C) N. retusa aqueous extract along with penconazole and (D) N. retusa aqueous extract. Optic microscopy: H&E (400×). Arrows indicate: Glomeruli fragmentation, necrosis of the epithelial cells lining the tubules, Bowman’s space enlargement, inflammatory leucocytes infiltration
Table 6.
Grading of the histopathological changes in the kidney sections.
| Groups | Enlarged Bowman’sspace | Glomerulifragmentation | Necrosis of the epithelial cellslining the tubules | Infiltration ofleucocytes |
|---|---|---|---|---|
| Controls | − | − | − | − |
| PEN | ++ | +++ | +++ | +++ |
| NRE + PEN | − | − | + | − |
| NRE | − | − | − | − |
Scoring was done as follows: none (−), mild (+), moderate (++) and severe (+++).
Discussion
In recent years, there is a growing concern worldwide over the indiscriminate use of pesticides, resulting in environmental pollution and toxicity risk to non-target organisms. Indeed, pesticides exposure has been associated with free radical-mediated damage in biological systems (Banerjee et al. 2001). This has prompted the intensive efforts to search for new antioxidant compounds, especially from natural sources, which are considered to be relatively safe and without side effects. Medicinal plants have been largely used in traditional medicine as remedies for many kinds of human diseases. The present study was undertaken to evaluate the potential toxic effects of penconazole on the kidney of adult rats and to investigate the possible protective effect of N. retusa fruit aqueous extract against toxicity induced by this fungicide.
In the present work, penconazole treatment had no effect on body weight as well as on food intake of adult rats, suggesting that this fungicide did not probably affect their appetite. Absolute and relative kidney weights were also unaffected following penconazole exposure. While, a significant rise in water consumption was recorded for penconazole-treated rats, which could be explained as a mechanism to counteract the toxicity induced by this fungicide.
Although there were no morphological changes including body or kidney weights in penconazole-treated rats, exposure to this fungicide caused alteration of renal function. Indeed, creatinine, creatinine clearance, urea and blood urea nitrogen (BUN) are used as indicators of renal function. Our results showed an increase of plasma levels in creatinine, urea, BUN and a reduced creatinine clearance in penconazole-treated rats, indicating renal dysfunction with a reduction in glomerular filtration rate. Increased plasma creatinine and BUN levels have been recorded by Takaori (1993) in rats fed with thiophanate-methyl, a benzimidazole fungicide, and by Badgujar et al. (2014) in rats exposed to fipronil, a phenyl pyrazole insecticide. This impairment in glomerular filtration rate was accompanied by an increase in 24-h urine volume, which might happen as a consequence of a reduced water reabsorption capacity by tubular epithelial cells.
Uric acid was another parameter used in the present work to assess kidney function. It is known for its antioxidant capacity based on its ability to scavenge free radicals (Alvarez-Lario & Macarron-Vicente 2010). An increase of plasma uric acid level after exposure to penconazole might reflect body response to an excess of ROS production, since uric acid is a potent free radicals scavenger. This finding is in agreement with the previous study of El-Demerdash et al. (2013) where plasma uric acid was found to be increased in the kidney of rats exposed to methomyl, a carbamate pesticide.
Administration of N. retusa aqueous extract to penconazole-treated rats attenuated the kidney impairment as suggested by a significant amelioration of the renal biomarkers indicated earlier. Nevertheless, we have noted a significant increase of urinary volume in rats receiving N. retusa aqueous extract alone, as compared to control rats. Our data indicated that this wild medicinal plant exerted probably a diuretic activity which could be due to its high salt content, as reported by us. In fact, N. retusa fruit contained high amounts of Na+, which could be due to its halophyte nature. Besides, according to Hegazy et al. (2013), this species fruit is rich in potassium (263.33 ± 6.11 mg/100 g NRFW), which is reported to have a diuretic effect (Nelson & Cox 2004). In addition, the presence of flavonoids in N. retusa fruit (Chaâbane et al. 2014) could explain the increase of diuresis as reported by Yuliana et al. (2009) who have indicated that flavonoids cause polyuria. Similar results have been found by Durairaj et al. (2007) concerning the effects of Oxystelma esculentum R. Br. (Asclepiadaceae) methanolic extract on diuresis in rats. Treatment of rats with N. retusa fruit aqueous extract induced also a significant rise in plasma urea and BUN values, which could be ascribed to the dehydratation caused by the diuretic effect of this extract, as indicated by Nayeem and Quadri (2015) in albino mice administered with Boswellia serrata Roxb. (Burseraceae) leaf extract.
As described previously, kidney function impairment could be attributed to oxidative damage probably caused by penconazole. It is well established that the kidney is highly vulnerable to the damage induced by ROS due to its high content of polyunsaturated fatty acids (Rodrigo & Rivera 2002). In the present study, penconazole induced lipid peroxidation in the kidney of adult rats as revealed by a marked elevation in MDA. Previous findings of Sakr (2007) have shown that treatment with mancozeb, another fungicide, induces a significant increase in lipid peroxidation in albino rats. The increased MDA level observed in tissues of animals exposed to penconazole could be probably ascribed to the excessive production of ROS caused by this fungicide leading to renal injury. In fact, the generation of the H2O2 was enhanced following treatment of rats with penconazole. Consistent with these observations, Poovala et al. (1998) have found an increase of H2O2 levels following exposure of renal tubular cells to acephate, an organophosphate insecticide. H2O2 generation has been involved in the pathogenesis of several forms of acute tubular injury, where the lipid peroxidation process plays an essential role (Salahudeen 1995). Mitochondria are considered to be the major cellular source for H2O2 production. Hence, it is suggested that penconazole, with lipophilic nature, could easily cross the cytoplasmic membrane to reach mitochondria, causing thereby the dysfunction of the mitochondrial respiratory chain and an enhanced generation of ROS including H2O2 which could be converted to a reactive hydroxyl radical. The latter can trigger a lipid peroxidation chain reaction by extracting an allylic hydrogen from polyunsaturated fatty acids.
As a consequence of lipid peroxidation, biological membranes are affected leading to the loss of their fluidity, a modification of their potential or an increase of their permeability leading to a cellular damage. In this regard, LDH, an intracellular enzyme, is recognized to be a potent marker for assessing chemical’s toxicity. This enzyme is released into the blood stream once the cell membrane integrity is disrupted in the conditions of oxidative stress (Zou et al. 2001). Our results demonstrated that, in penconazole-treated rats, LDH activity decreased significantly in kidney tissues while it increased in plasma when compared to controls. This observation might be attributed to a generalized increase in membrane permeability as a result of lipid peroxidation under ROS generation by penconazole.
In addition to LDH, ALP and GGT, which are membrane bound enzymes, can be also used as the reliable biomarkers of cell and tissue damage induced by toxic substances. In the current study, penconazole exposure resulted in a significant decline of plasma ALP activity which might be attributed to a decreased osteoblastic activity in bone, since ALP is formed in the osteoblasts. Hence, the bone tissue could be affected by penconazole exposure. Our results showed also a significant reduction in plasma GGT activity in penconazole-treated rats which might be due to the occurrence of oxidative stress through an excessive generation of ROS, as reported by Abdelhalim and Moussa (2013) in rats exposed to gamma radiation. Likewise, Abd-Alrahman et al. (2014) have demonstrated that exposure of rats to difenoconazole, another triazole fungicide, decreases plasma GGT activity.
Moreover, ROS can also directly attack cellular proteins leading to a severe failure of their biological functions and even cell death. Among the various oxidative modifications of amino acids in proteins, PCO formation may be an early marker for protein oxidation. Indeed, the process of protein carbonylation is nonreversible, causing conformational changes, decreases enzyme catalytic activities, resulting in the breakdown of proteins by proteases (Zhang et al. 2008). In addition, the degree of protein damage in experimental rats can be estimated by a novel marker, AOPP. Under our experimental conditions, the increased PCO and AOPP levels were detected in the kidney of penconazole-treated rats suggesting that oxidative protein damage might be one of the mechanisms underlying penconazole-induced nephrotoxicity. Previous studies have shown that pesticides exposure may promote protein oxidation in the kidney of adult rats (Ben Amara et al. 2013).
Treatment with N. retusa aqueous extract prevented oxidative damage induced by penconazole in the renal tissue, objectified by a significant decrease in the levels of MDA, H2O2, PCO and AOPP, as well as an improvement of ALP and GGT activities. This might be attributed to the free radical scavenging property of the plant extract.
Defence of renal cells against oxidative stress is maintained by several mechanisms including enzymatic and non-enzymatic antioxidants. Antioxidant enzymes such as CAT, SOD and GPx present an impressive array of defence mechanisms, since they are effective in maintaining ROS under adequate concentrations. SOD accomplishes its antioxidant function by catalyzing the dismutation of the superoxide radical () to H2O2, protecting thereby the kidney from damage. The production of the potentially harmful H2O2 is counteracted by the action of CAT and GPx, catalyzing the decomposition of this ROS to water and oxygen. In the current study, penconazole treatment provoked changes in renal enzymatic antioxidant systems as confirmed by the increased activities of CAT, SOD and GPx. The increment in the activities of these enzymes might be understood as an adaptive response of the body to oxidative stress as reported by Sengupta et al. (1990) in rats exposed to chromium.
As antioxidant enzymes work in synergy with non-enzymatic antioxidants, it was necessary to evaluate renal GSH and NPSH levels. GSH is thought to be an important endogenous defence molecule against peroxidative destruction of cellular membranes. This tripeptide can react directly with ROS and electrophilic metabolites and protects essential thiol groups from oxidation. In our study, significant rises in kidney GSH and NPSH levels were noted following penconazole treatment, which might occur as a mechanism of protection against oxidative damage.
Cellular defence against oxidative stress is also induced by metallothionein (MT), a small metal-binding and cysteine-rich protein. Its high thiol content makes it a powerful OH• scavenger more efficient than GSH (Deneke 2000). This protein has been widely used to identify specific responses to heavy metals pollution. MT synthesis can be also induced by compounds other than heavy metals leading to ROS production. In our study, penconazole stimulated the kidney to produce MT as illustrated by its significant increase when compared to controls. This could be considered as a defence mechanism, in addition to the antioxidants, against oxidative stress and the adverse effects induced by this fungicide.
Interestingly, treatment with N. retusa aqueous extract improved the enzymatic and non-enzymatic antioxidant status of rats exposed to penconazole, indicating that this plant extract participated in the reduction of penconazole toxicity by decreasing oxidative stress due to the ability of its antioxidant compounds to scavenge free radicals.
It is worth mentioning that, in a previous study, we have shown that the aqueous extract of N. retusa fruit exhibited a significant protective effect against penconazole-induced oxidative stress in the liver of adult rats (Chaâbane et al. 2015). Based on our previous and present findings, penconazole toxicity in rats seems to be more pronounced in the liver than in the kidney. Indeed, penconazole treatment increased the hepatic levels of MDA, H2O2, AOPP, NPSH, MT, SOD, CAT and GPx by 103, 56, 181, 23, 17, 36, 52 and 52%, respectively. In line with this, tests on short-term toxicity have shown that the liver is the main target organ for this fungicide (EFSA 2008). As for the beneficial effect of N. retusa extract administration on penconazole toxicity, this plant extract showed to be more effective in reducing the elevation of MDA (−41%), H2O2 (−14%), AOPP (−44%), NPSH (−10%) and CAT (−22%) levels caused by penconazole in the liver of adult rats than in the renal tissue. While, it was found to be less successful in decreasing the rise of MT (−8%), SOD (−9%) and GPx (−13%) levels induced by penconazole in the hepatic tissue as compared to the present findings. So, the hepatoprotective potential of N. retusa extract appears to be more potent than its nephroprotective activity, especially in regards to its modulatory effects on lipid and protein oxidation.
The biochemical findings obtained in the current work were substantiated with histopathological observations. The renal tubules as well as the glomeruli were affected after penconazole treatment. In fact, kidney sections of penconazole-treated rats showed a renal tubular damage characterized by necrosis of the epithelial cells lining the tubules. This fungicide caused also an infiltration of leucocytes. These observations could be due to the accumulation of free radicals as a consequence of a lipid peroxidation increase in the renal tissue of penconazole-treated rats. Similar histopathological observations have been reported in kidney of rats treated with the metiram, another fungicide (Sakr et al. 2013). Moreover, kidney of rats exposed to penconazole showed glomeruli fragmentation and an enlarged Bowman’s space which matched with the previous findings of Shah and Iqbal (2010) in diazinon-treated rats.
Kidney histoarchitecture impairment, induced by penconazole, was remarkably reduced by the aqueous extract of N. retusa fruit confirming, consequently, the biochemical results.
Polyphenol constituents of N. retusa fruit aqueous extract might participate with their antioxidant activities in nephroprotection. In fact, the analysis of this extract by HPLC-HRMS is the first study which identified the phenolic compounds, including phenolic acids and flavonoids, whose major components were (3′-O-methyl-(-)-epicatechin 7-O-glucuronide, 4′-O-methyl-(-)-epicatechin 3′-O-glucuronide, epicatechin 3′-O-glucuronide, p-coumaric acid, cyanidin 3-O-rutinoside, 3-O-methylgallic acid, taxifoline and kaempferol 3-glucoside). They have been reported for their powerful antioxidant activity (Natsume et al. 2004; Luceri et al. 2007; Mulabagal et al. 2007; Gargouri et al. 2013; Topal et al. 2015; Wang et al. 2015). Furthermore, the nephroprotective effect of chlorogenic acid against cisplatin-induced kidney injury has been previously reported by Domitrović et al. (2014). In addition, Vijayaprakasha et al. (2013) have shown that kaempferol alleviates the toxicity induced by mercuric chloride in the kidney of rats. According to Zadernowski et al. (2005), hydroxycaffeic acid has been found to be the major phenolic acid in blackberries but information regarding its biological activities seems to be lacking. In general, polyphenols have been shown to attenuate renal dysfunction, to decrease lipid peroxidation and ROS, and to improve renal histoarchitecture (Wongmekiat et al. 2008). The renoprotective effect of phenolic compounds is thought to be essentially due to their wide range of biological actions, such as free radical scavenging, metal chelation and enzyme modulation capacities (Pietta et al. 1998). In particular, flavonoids represent an important class of phenolic compounds and have been reported to inhibit xenobiotic-induced nephrotoxicity in animal models (Devipriya & Shyamaladevim 1999). They exhibit a potent antioxidant effect, which is attributed to their free radical scavenging properties, their ability to reduce free radicals formation (Pietta 2000) and to stabilize membranes by decreasing their fluidity (Arora et al. 2000), which may inhibit the lipid peroxidation process. Furthermore, the mineral composition of N. retusa fruit could contribute to its antioxidant property. Trace elements such as Fe2+, Zn2+ and Cu2+ have been reported to play the key roles as cofactors for endogenous antioxidant enzymes (Powell 2000; Michalak 2006). The biological activities of these microelements are related to the presence of unpaired electrons allowing their participation in redox reactions.
Overall, our results suggested that the main mechanism underlying penconazole nephrotoxicity in rats involve the generation of ROS, resulting in the subsequent formation of oxidative stress in the renal tissue. According to the literature data, increased production of ROS can activate redox sensitive transcription factors, such as nuclear factor-κB (NF-κB), and signal transduction pathways, like mitogen-activated protein kinases (MAPKs). These events may lead to tissue damage and dysfunction by promoting several disorders such as necrosis, apoptosis, inflammation and fibrosis. Indeed, NF-κB is a ubiquitous transcription factor governing the expression of genes encoding a series of proinflammatory cytokines and chemokines. Its activation plays, therefore, a pivotal role in the pathogenesis of inflammation, tissue injury and renal disease (An et al. 2009). MAPKs represent a family of structurally related serine/threonine kinase enzymes, which include the extracellular-receptor kinases (ERK1/2), the c-Jun N-terminal kinases (JNK) and the p38 MAPKinases. The MAPKs pathway operates a crucial signalling pathway of cell death and inflammation in kidney (Xu et al. 2014). In this context, it has been demonstrated, in animal models of diabetic nephropathy, that ROS mediate the activation of various signalling pathways induced by high glucose, including MAPKs and NF-κB, and up-regulate genes and proteins involved in glomerular mesangial expansion and tubule-interstitial fibrosis (Lee et al. 2003). On the other hand, the nuclear factor erythroid-2-related factor (Nrf2), another transcription factor, is considered to be a main defence mechanism against oxidative stress in cells (Li et al. 2008). Nrf2 expression and its nuclear translocation have been involved in the induction of antioxidant defences and phase II detoxifying enzymes (Xu et al. 2014). In an experimental model of acute kidney injury in mice, Sahu et al. (2015) found that baicalein, a bioflavonoid, prevents cisplatin-induced renal injury by up-regulating Nrf2 expression and down-regulating the MAPKs and NF-κB pathways. Hence, to improve our understanding of the molecular mechanisms of penconazole-induced nephrotoxicity, it would be interesting to examine, in the future studies, the target pathways activated by ROS in renal cells exposed to this fungicide and the protective effects of N. retusa fruit extract.
Conclusions
Our study provided a new knowledge regarding the phenolic composition of N. retusa fruit aqueous extract which showed to be a source of phenolic acids and flavonoids. Moreover, we demonstrated the beneficial contribution of this extract in mitigating penconazole-induced nephrotoxicity in adult rats. Therefore, N. retusa fruit aqueous extract could be used as a potential food supplement for the treatment of penconazole-induced kidney injury.
Funding Statement
This work was supported by the Ministry of Higher Education and Scientific Research [04/UR/09-04], Tunisia.
Acknowledgements
Our grateful thanks were addressed to Professors Mohamed Chaieb and Ferjani Ben Abdallah who have identified the plant.
The authors are grateful to Mr. Chedli Tmar for the laboratory animal’s maintenance and Mrs Raoudha Ben Amar Abdennadher for her skillful technical assistance.
Disclosure statement
The authors report no declarations of interest.
References
- Abdelhalim MAK, Moussa SAA. 2013. The biochemical changes in rats' blood serum levels exposed to different gamma radiation doses. Afr J Pharm Pharmacol. 7:785–792. [Google Scholar]
- Abd-Alrahman SH, Elhalwagy MEA, Kotb GA, Farid H, Farag AAG, Draz HM, Isa AM, Sabico S. 2014. Exposure to difenoconazole, diclofop-methyl alone and combination alters oxidative stress and biochemical parameters in albino rats. Int J Clin Exp Med. 7:3637–3646. [PMC free article] [PubMed] [Google Scholar]
- Aebi H. 1984. Catalase in vitro . Meth Enzymol. 105:121–126. [DOI] [PubMed] [Google Scholar]
- Ahmed NS, Mohamed AS, Abdel-Wahhab MA. 2010. Chlorpyrifos induced oxidative stress and histological changes in retinas and kidney in rats: protective role of ascorbic acid and α tocopherol. Pestic Biochem Phys. 98:33–38. [Google Scholar]
- Alvarez-Lario B, Macarron-Vicente J. 2010. Uric acid and evolution. Rheumatology. 49:2010–2015. [DOI] [PubMed] [Google Scholar]
- An WS, Kim HJ, Cho KH, Vaziri ND. 2009. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubule-interstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol. 297:895–903. [DOI] [PubMed] [Google Scholar]
- Arora A, Byrem TM, Nair MG, Stasburg GM. 2000. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys. 373:102–109. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 2000. Official methods of analysis. 17th ed Gaithersburg, MD, USA: AOAC International. [Google Scholar]
- Badgujar PC, Pawar NN, Chandratre GA, Telang AG, Sharma AK. 2014. Fipronil induced oxidative stress in kidney and brain of mice: protective effect of vitamin E and vitamin C. Pest Biochem Physiol. 118:10–18. [DOI] [PubMed] [Google Scholar]
- Banerjee BD, Seth V, Ahmed RS. 2001. Pesticide-induced oxidative stress: perspectives and trends. Rev Environ Health. 16:1–40. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. 2004. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 3:205–214. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 44:276–287. [DOI] [PubMed] [Google Scholar]
- Ben Amara I, Karray A, Hakim A, Ben Ali Y, Troudi A, Soudani N, Boudawara T, Zeghal KM, Zeghal N. 2013. Dimethoate induces kidney dysfunction, disrupts membrane-bound ATPases and confers cytotoxicity through DNA damage. Protective effects of vitamin E and selenium. Biol Trace Elem Res. 156:230–242. [DOI] [PubMed] [Google Scholar]
- Benvenuti S, Pellati F, Melegari M, Bertelli D. 2004. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J Food Sci. 69:164–169. [Google Scholar]
- Bhinder P, Chaudhry A. 2013. Mutagenicity assessment of organophosphates using polymerase chain reaction-restriction fragment length polymorphism assay. Toxicol Int. 20:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabras P, Angioni A. 2000. Pesticide residues in grapes, wine, and their processing products . J Agric Food Chem. 48:967–973. [DOI] [PubMed] [Google Scholar]
- Chaâbane M, Maktouf S, Sayari N, Zouari S, Zeghal N, Ellouze Ghorbel R. 2014. Antioxidant and antimicrobial properties of the extracts from Nitraria retusa fruits and their applications to meat product preservation. Ind Crops Prod. 55:295–303. [Google Scholar]
- Chaâbane M, Soudani N, Benjeddou K, Turki M, Ayadi Makni F, Boudawara T, Zeghal N, Ellouze Ghorbel R. 2015. The protective potential of Nitraria retusa on penconazole-induced hepatic injury in adult rats. Toxicol Environ Chem. 97:1253–1264. [Google Scholar]
- Chaâbane M, Tir M, Hamdi S, Boudawara O, Jamoussi K, Boudawara T, Ellouze Ghorbel R, Zeghal N, Soudani N. 2016. Improvement of heart redox states contributes to the beneficial effects of selenium against penconazole-induced cardiotoxicity in adult rats. Biol Trace Elem Res. 169:261–270. [DOI] [PubMed] [Google Scholar]
- Charrel M. 1991. Urée et créatinine. In: Sémiologie biochimique (Urea and creatinin). Paris: Ellipses Publishers; p. 124–128. [Google Scholar]
- Council of European Communities 1986. Council instructions about the protection of living animals used in scientific investigations. OJEC (Jo 86/609/Cee). 358:1–18. [Google Scholar]
- Deneke SM. 2000. Thiol-based antioxidants. Curr Top Cell Regul. 36:151–180. [DOI] [PubMed] [Google Scholar]
- Devipriya S, Shyamaladevim CS. 1999. Protective effect of quercetin in cisplatin induced cell injury in the rat kidney. Indian J Pharmacol. 31:422–426. [Google Scholar]
- Domitrović R, Cvijanović O, Šušnić V, Katalinić N. 2014. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology. 324:98–107. [DOI] [PubMed] [Google Scholar]
- Draper HH, Hadley M. 1990. Malondialdehyde determination as index of lipid peroxidation . Meth Enzymol. 186:421–431. [DOI] [PubMed] [Google Scholar]
- Durairaj AK, Mazumder UK, Gupta M, Ray SK. 2007. Effects of methanolic extract of Oxystelma esculentum on diuresis and urinary electrolytes excretion in rats. Iran J Pharmacol Ther. 6:207–211. [Google Scholar]
- European Food Safety Authority (EFSA) 2008. Conclusion regarding the peer review of the pesticide risk assessment of the active substance penconazole. EFSA Sci Rep. 175:1–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Demerdash F, Dewer Y, El Mazoudy RH, Attia AA. 2013. Kidney antioxidant status, biochemical parameters and histopathological changes induced by methomyl in CD-1 mice. Exp Toxicol Pathol. 65:897–901. [DOI] [PubMed] [Google Scholar]
- Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys. 82:70–77. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy EE, El-Nisr NA. 2013. Testicular dysfunction induced by penconazole fungicide on male albino rats. Comp Clin Pathol. 22:475–480. [Google Scholar]
- Flohe L, Gunzler WA. 1984. Assays of glutathione peroxidase. Methods Enzymol. 105:114–121. [DOI] [PubMed] [Google Scholar]
- Galal MK, Khalaf AAA, Ogaly HA, Ibrahim MA. 2014. Vitamin E attenuates neurotoxicity induced by deltamethrin in rats. BMC Complement Altern Med. 14:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri OD, Gargouri B, Trabelsi SK, Bouaziz M, Abdelhedi R. 2013. Synthesis of 3-O-methylgallic acid a powerful antioxidant by electrochemical conversion of syringic acid. Biochim Biophys Acta. 1830:3643–3649. [DOI] [PubMed] [Google Scholar]
- Goetz AK, Ren HZ, Schmid JE, Blystone CR, Thillainadarajah I, Best DS, Nichols HP, Strader LF, Wolf DC, Narotsky MG, et al. 2007. Disruption of testosterone homeostasis as a mode of action for the reproductive toxicity of triazole fungicides in the male rat. Toxicol Sci. 95:227–239. [DOI] [PubMed] [Google Scholar]
- Greim H, Saltmiras D, Mostert D, Strupp C. 2015. Evaluation of carcinogenic potential of the herbicide glyphosate, drawing on tumor incidence data from fourteen chronic/carcinogenicity rodent studies. Crit Rev Toxicol. 45:185–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy AK, Al-Rowaily SL, Faisal M, Alatar AA, El-Bana MI, Assaeed AM. 2013. Nutritive value and antioxidant activity of some edible wild fruits in the Middle East. J Med Plants Res. 15:938–946. [Google Scholar]
- Hou B, Wu L. 2010. Safety impact and farmer awareness of pesticide residues. Food Agric Immunol. 21:191–200. [Google Scholar]
- Hou Y, Zeng Y, Li S, Qi L, Xu W, Wang H, Zhao X, Sun C. 2014. Effect of quercetin against dichlorvos induced nephrotoxicity in rats. Exp Toxicol Pathol. 66:211–218. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. 1974. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 11:151–169. [DOI] [PubMed] [Google Scholar]
- Kalendar S, Kalendar Y, Durak D, Ogutcu A, Uzunhisarcikli M, Cevrimli BS, Yildirim M. 2007. Methyl parathion induced nephrotoxicity in male rats and protective role of vitamins C and E. Pest Biochem Physiol. 88:213–218. [Google Scholar]
- Kayali R, Cakatay U, Akcay T, Altug T. 2006. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct. 24:79–85. [DOI] [PubMed] [Google Scholar]
- Koubaa M, Mhemdi H, Vorobiev E. 2015. Seed oil polyphenols: rapid and sensitive extraction method and high resolution-mass spectrometry identification. Anal Biochem. 476:91–93. [DOI] [PubMed] [Google Scholar]
- Le Floc’h E. 1983. Contribution à une étude Ethnobotanique de la Flore Tunisienne 2nd ed Ministère de l’enseignement Supérieur et de la Recherche Scientifique; Imprimerie Officielle de la République Tunisienne, Tunis, p. 136–137. [Google Scholar]
- Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. 2003. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 14:241–245. [DOI] [PubMed] [Google Scholar]
- Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. 2008. Activation of Nrf2-antioxidant signaling attenuates NF-κB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 76:1485–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrugh NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275. [PubMed] [Google Scholar]
- Luceri C, Giannini L, Lodovici M, Antonucci E, Abbate R, Masini E, Dolara P. 2007. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br J Nutr. 97:458–468. [DOI] [PubMed] [Google Scholar]
- Michalak A. 2006. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud. 15:523–530. [Google Scholar]
- Mulabagal V, van Nocker S, Dewitt DL, Nair MG. 2007. Cultivars of apple fruits that are not marketed with potential for anthocyanin production. J Agric Food Chem. 55:8165–8169. [DOI] [PubMed] [Google Scholar]
- Natsume M, Osakabe N, Yasuda A, Baba S, Okunaga T, Kondo K, Osawa T, Terao J. 2004. In vitro antioxidative activity of (-)-epicatechin glucuronide metabolites present in human and rat plasma . Free Radic Res. 38:1341–1348. [DOI] [PubMed] [Google Scholar]
- Nayeem M, Quadri MFA. 2015. Evaluation of diuretic activity of Boswellia serrata leaf extracts in albino mice. Int J Pharm Pharm Sci. 7:502–505. [Google Scholar]
- Nelson DL, Cox MM. 2004. Lehninger Principles of Biochemistry. Sao Paulo: Sarvier; p. 1304. [Google Scholar]
- Ogutcu A, Suludere Z, Kalender Y. 2008. Dichlorvos-induced hepatotoxicity in rats and the protective effects of vitamins C and E. Environ Toxicol Pharmacol. 26:355–361. [DOI] [PubMed] [Google Scholar]
- Ou P, Wolff SP. 1996. A discontinuous method for catalase determination at near physiological concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J Biochem Biophys Methods. 31:59–67. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Siedlak SL, Harris PLR, Xiongwei Z, Castellani RJ, Aliev G, Smith MA. 2001. Oxidant and antioxidant responses in Alzheimer disease. Rec Res Dev Biophys Biochem. 1:35–41. [Google Scholar]
- Petrovic S, Ozretic B, Krajnovic-Ozretic M, Bobinac D. 2001. Lysosomal membrane stability and metallothioneins in digestive gland of mussels (Mytilus galloprovincialis Lam.) as biomarkers in a field study. Mar Pollut Bull. 42:1373–1378. [DOI] [PubMed] [Google Scholar]
- Pietta P, Simonetti P, Gordana C, Brusamolino A, Morazzoni P, Bombardelli E. 1998. Relationship between rate and extent of catechin absorption and plasma antioxidant status. Biochem Mol Biol Int. 46:895–903. [DOI] [PubMed] [Google Scholar]
- Pietta PG. 2000. Flavonoids as antioxidants. J Nat Prod. 63:1035–1042. [DOI] [PubMed] [Google Scholar]
- Poovala VS, Vijaya KK, Tachikawa H, Salahudeen AK. 1998. Role of oxidant stress and antioxidant protection in acephate-induced renal tubular cytotoxicity. Toxicol Sci. 46:403–409. [DOI] [PubMed] [Google Scholar]
- Pottier-Alapetite G. 1979. Flowers of Tunisia: Angiosperms, dicotyledons, apetals, dialypetals Tunisia. Tunisia: Ministry of Higher Education and Scientific Research and the Ministry of Agriculture; p. 456. [Google Scholar]
- Powell SR. 2000. The antioxidant properties of zinc. J Nutr. 130:1447–1454. [DOI] [PubMed] [Google Scholar]
- Reznick AZ, Packer L. 1994. Oxidative damage to proteins: spectrophotometric method for carbonyl Methods Enzymol. New York: Academic Press; p. 357–359. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Bolwell PG, Bramlev PM, Pridham JB. 1995. The relative antioxidant activities of plant-derived polyphenolic flavonoids . Free Radic Res. 22:375–383. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Rivera G. 2002. Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radic Biol Med. 33:409–422. [DOI] [PubMed] [Google Scholar]
- Sahu BD, Kumar JM, Sistla R. 2015. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLOS One. 10:e0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S, EL-Kenawy A, EL-Sahra D. 2013. Metiram-induced nephrotoxicity in albino mice: effect of licorice aqueous extract. Environ Toxicol. 28:372–379. [DOI] [PubMed] [Google Scholar]
- Sakr SA. 2007. Ameliorative effect of ginger (Zingiber officinale) on mancozeb fungicide induced liver injury in albino rats. Aust J Basic Appl Sci. 1:650–656. [Google Scholar]
- Salahudeen AK. 1995. Role of lipid peroxidation in H2O2-induced renal epithelial (LLC-PK1) cell injury. Am J Physiol. 268:30–38. [DOI] [PubMed] [Google Scholar]
- Sengupta T, Chattopadhay D, Ghosh N, Das M, Chatterjee GC. 1990. Effect of chromium administration on glutathione cycle of rat intestinal epithelial cells. Indian J Exp Biol. 28:1132–1135. [PubMed] [Google Scholar]
- Shah MD, Iqbal M. 2010. Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem Toxicol. 48:3345–3353. [DOI] [PubMed] [Google Scholar]
- Shaltout KH, Sheded MG, El-Kady HF, Al-Sodany YM. 2003. Phytosociology and size structure of Nitraria retusa along the Egyptian Red Sea coast. J Arid Environ. 53:331–345. [Google Scholar]
- Takaori H. 1993. Thiophanate-methyl combined chronic toxicity/oncogenicity study in rats. Unpublished report no.RD-9327 from Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan. [Google Scholar]
- Ting CM, Lee YM, Wong CKC, Wong AS, Lung HL, Lung ML, Lo KW, Wong RN, Mak NK. 2010. 2-Methoxyestradiol induces endoreduplication through the induction of mitochondrial oxidative stress and the activation of MAPK signaling pathways. Biochem Pharmacol. 79:825–841. [DOI] [PubMed] [Google Scholar]
- Topal F, Nar M, Gocer H, Kalin P, Kocyigit UM, Gülçin I, Alwasel SH. 2015. Antioxidant activity of taxifolin: an activity–structure relationship. J Enzyme Inhib Med Chem. 6:1–10. [DOI] [PubMed] [Google Scholar]
- Viarengo A, Ponzano E, Dondero F, Fabbri R. 1997. A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res. 44:69–84. [Google Scholar]
- Vijayaprakasha S, Langeswaranb K, Kumar SG, Revathya R, Balasubramaniana MP. 2013. Nephro-protective significance of kaempferol on mercuric chloride induced toxicity in Wistar albino rats. Biomed Aging Pathol. 3:119–124. [Google Scholar]
- Wang Y, Tang C, Zhang H. 2015. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J Food Drug Anal. 23:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongmekiat O, Leelarugrayub N, Thamprasert K. 2008. Beneficial effect of shallot (Allium ascalonicum L.) extract on cyclosporine nephrotoxicity in rats. Food Chem Toxicol. 46:1844–1850. [DOI] [PubMed] [Google Scholar]
- Xavier R, Rekha K, Bairy KL. 2004. Health perspective of pesticide exposure and dietary management. Malays J Nutr. 10:39–51. [PubMed] [Google Scholar]
- Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, Wie W, Lu X. 2014. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway . Free Radic Biol Med. 71:186–195. [DOI] [PubMed] [Google Scholar]
- Xu W, Shao X, Tian L, Gu L, Zhang M, Wang Q, Wu B, Wang L, Yao J, Xu X, et al. 2014. Astragaloside IV ameliorates renal fibrosis via the inhibition of mitogen-activated protein kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp Ther. 350:552–562. [DOI] [PubMed] [Google Scholar]
- Yuliana ND, Khatib A, Link-Struensee AM, Ijzerman AP, Rungkat-Zakaria F, Choi YH, Verpoorte R. 2009. Adenosine A1 receptor binding activity of methoxy flavonoids from Orthosiphon stamineus. Planta Med. 75:132–136. [DOI] [PubMed] [Google Scholar]
- Zadernowski R, Naczk M, Nesterowicz J. 2005. Phenolic acid profiles in some small berries. J Agric Food Chem. 53:2118–2124. [DOI] [PubMed] [Google Scholar]
- Zarn JA, Bruschweiler BJ, Schlatter JR. 2003. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ Health Perspect. 111:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang F, Zhang X, Xu Y, Liao T, Song S, Wang H. 2008. Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD). Aquat Toxicol. 86:4–11. [DOI] [PubMed] [Google Scholar]
- Zou W, Yan M, Xu W, Huo H, Sun L, Zheng Z. 2001. Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J Neurosci Res. 64:646–653. [DOI] [PubMed] [Google Scholar]