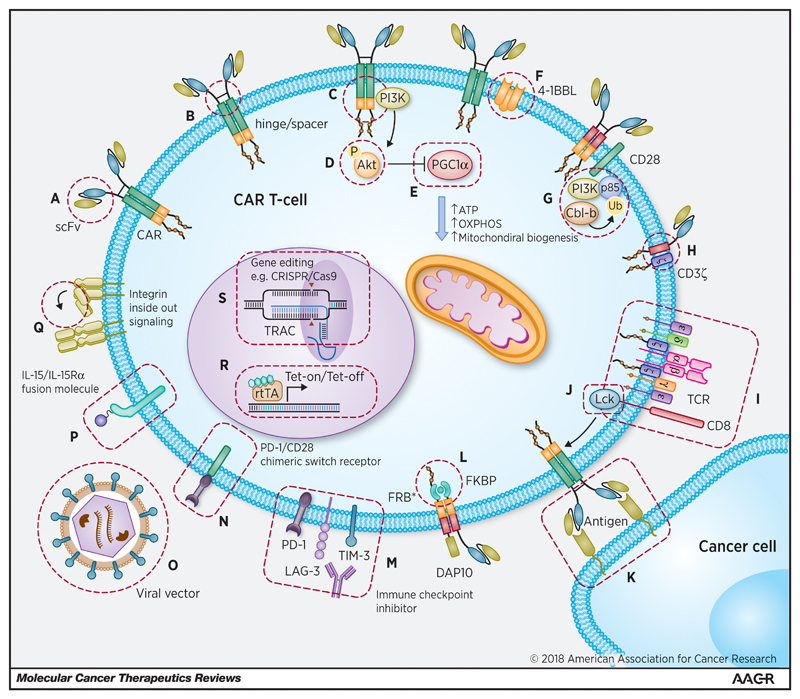
Figure 5.
Potential strategies to address the negative effects of CAR tonic signalling. (a) Optimal selection of the extracellular targeting moiety +/- engineering of the scFv or substitution with camelid-derived nanobodies or non-immunoglobulin based scaffolds; (b) optimisation of the hinge and spacer; (c) optimal selection of costimulatory endodomains; (d) utilising pharmacological agents to reverse or prevent negative consequences of tonic signalling (e.g. Akt inhibitors to prevent terminal effector differentiation +/- metabolic features of T-cell exhaustion); (e) engineering CAR T-cell metabolism (e.g. overexpressing PGC1α or impairing its degradation; (f) reconfiguring costimulation by overexpressing costimulatory molecules or ligands such as 4-1BBL or (g) CD28, potentially optimised by knocking down expression of Cbl-b, an E3 ubiquitin-protein ligase, that promotes anergy by regulating PI3K access to CD28; (h) optimising interactions with endogenous TCR components, which may contribute to CAR tonic signalling; (i) recapitulating or enhancing T-cell / DC interactions to lower the activation threshold for cytotoxicity; (j) preventing constitutive IL-2 production and Treg induction by mutating the CD28 binding site for Lck; (k) optimal selection of target ligand, autologous APCs, T-APCs or EATCs expressing target ligand may also facilitate CAR T-cell expansion and persistence in vivo; (l) utilising small molecule gated CARs e.g. by incorporating an FKBP/FRB* heterodimerizing module in the presence of a rapamycin analogue; (m) utilising blocking monoclonal antibodies to target inhibitory immune checkpoints; (n) utilising switch CARs (e.g. PD-1/CD28); (o) optimal selection of the expression vector and promoter, e.g. using non-LTR (SIN) lentiviruses, mRNA or transposon delivery; (p) co-expressing tethered cytokine fusion molecules (such as IL-15/IL-15Rα); (q) exploiting inside-out signalling to integrins to facilitate T-cell migration & bystander tumour cell targeting; (r) utilising Tet-off systems for temporal control of CAR expression; and (s) utilising CRISPR Cas9 to direct CAR expression specifically to the T-cell receptor α constant (TRAC) locus.
Figure 1 and figures 3-5 are original and have been created specifically for this article.