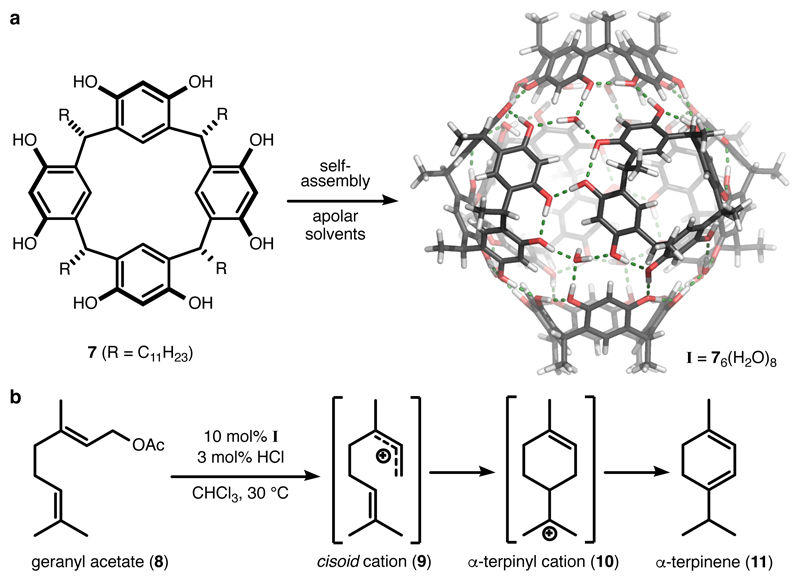
Figure 2. Selective cyclisation of the monoterpene geranyl acetate catalysed by the resorcinarene capsule.
a, The resorcinarene monomers 7 self-assemble in apolar solvents with eight water molecules to form the hexameric capsule I with an internal volume of 1.4 nm3. The capsule is capable of stabilizing cationic species via cation-π interactions. b, The resorcinarene capsule was employed as an artificial terpene cyclase mimic for the cyclisation of monoterpenes. The cyclisation reactions were likely facilitated by the stabilisation of the cationic intermediates/transition states by the capsule.