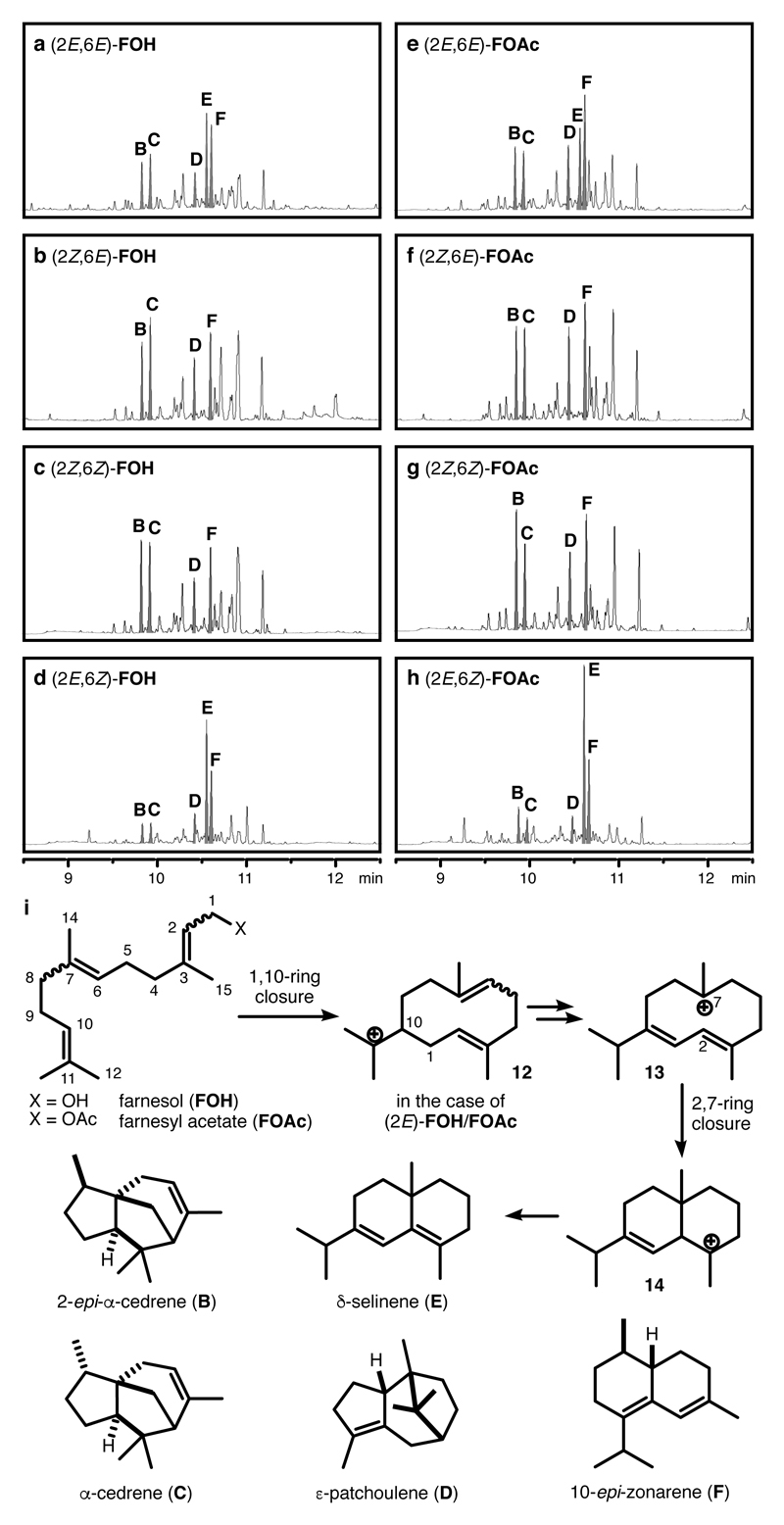
Figure 3. Product analysis of the capsule-catalysed cyclisation reactions of farnesyl substrates.
a-h, GC-traces of the cyclisation reaction of (2E,6E)-FOH, (2Z,6E)-FOH, (2Z,6Z)-FOH, (2E,6Z)-FOH, (2E,6E)-FOAc, (2Z,6E)-FOAc, (2Z,6Z)-FOAc and (2E,6Z)-FOAc at full conversion of substrates. The intensity of the peaks in the GC-traces are normalized to an internal standard (For full spectra, see Supplementary Figure 1-8) i, the structures of the cyclisation precursors, the main cyclisation products and the proposed mechanism from (2E)-FOH/FOAc to δ–selinene (E). The double bond geometry in the substrate accounts for the different product selectivities observed. The higher selectivity of the cyclisation reaction of (2E,6Z)-FOAc is attributed to the conformational control of the substrate and the reaction intermediate.