Fig. 1. MDMX is targetable in acute myeloid leukemia using an a-helical stapled peptide (ALRN-6924).
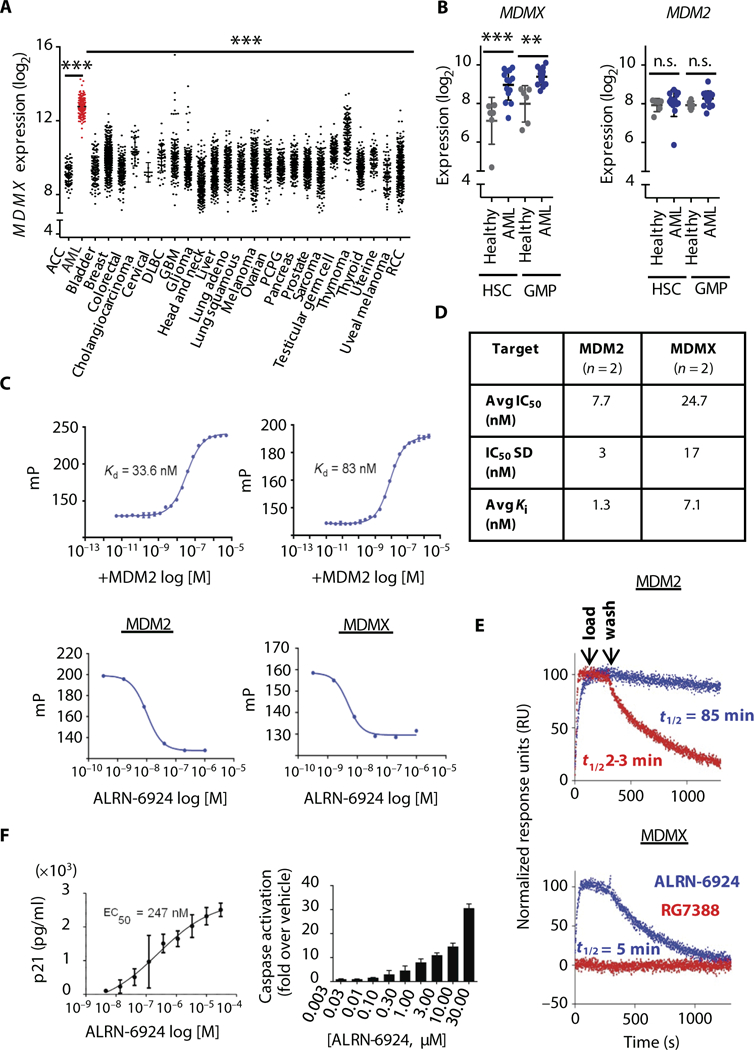
(A) mRNA expression analysis of MDMX in human cancers using The Cancer Genome Atlas (TCGA) data sets and (B) MDMX and MDM2 mRNA expression in hematopoietic stem (HSC) and granulocyte-monocytic progenitor-enriched (GMP) cells from acute myeloid leukemia (AML) patients (n = 14) and healthy controls (n = 6) (data shown as mean ± SD, where **P < 0.01, ***P < 0.001; n.s., not significant). (C) Fluorescence polarization (mP) of a fixed concentration of ALRN-3618 was measured in the presence of varying concentrations (log M) of mouse double minute 2 homolog (MDM2) and MDMX (top panels) (n = 2). Graphs display varying concentrations of ALRN-6924 used to displace ALRN-3618 at its EC80 from each target protein (bottom panels). (D) IC50 and Kl values (n = 2) for each target protein, as determined from a nonlinear four-parameter curve obtained from plotting fluorescence polarization versus drug concentration in (C) (bottom). (E) Sensorgrams for the binding of ALRN-6924 (blue) and RG7388 (red) to surfaces with immobilized MDM2 (top) and MDMX (bottom) (n = 2). (F) Enzyme-linked immunosorbent assay demonstrating the expression of human p21 protein (left) (21 hours after treatment with ALRN-6924) and cellular caspase activity (right) (48 hours after treatment with ALRN-6924) determined using Caspase-Glo 3/7 assay reagent in SJASA-1 cells (n = 2; data shown as mean ± SD). ACC, adrenocortical carcinoma; DLBC, diffuse large B cell lymphoma; PCPG, pheochromocytoma and paraganglioma; RCC, renal cell carcinoma.
