Fig. 6. ALRN-6924 selectively activates p53 in leukemia cells of an MDS/AML patient in vivo.
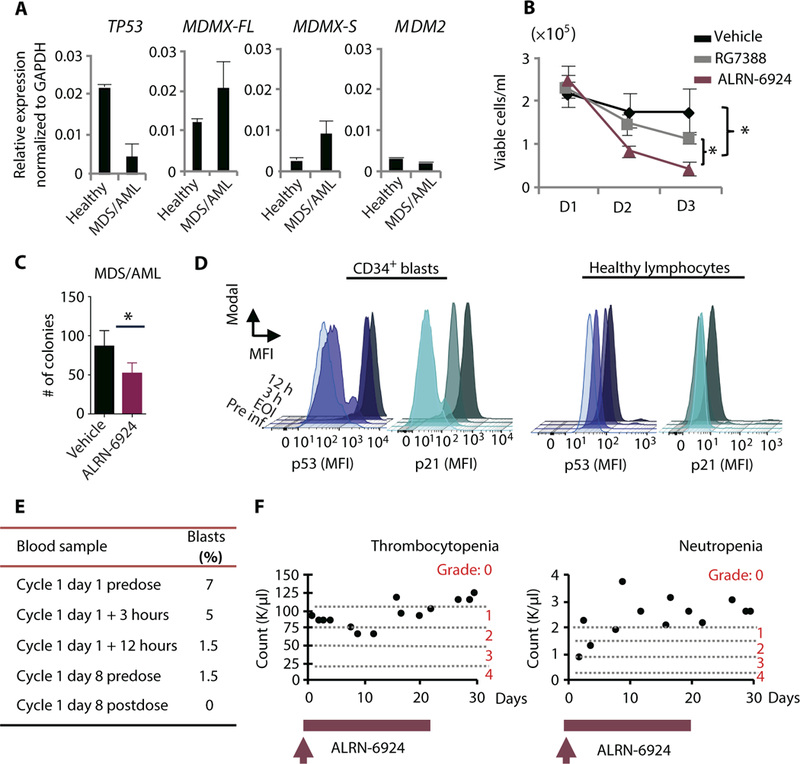
(A) mRNA expression analysis by qRT-PCR of TP53, MDMX-FL, MDMX-S, and MDM2 in leukemic cells isolated from peripheral blood of a patient with myelodysplastic syndrome (MDS)/AML. (B) Cell viability assay using patient-derived leukemic PBMNCs evaluated by trypan blue exclusion. PBMNCs were treated in liquid culture with vehicle, 10 μM ALRN-6924, or 10 μM RG7388 and monitored over time. (C) CFC assay using patient-derived leukemic cells treated with vehicle or 0.5 μM ALRN-6924 as in Fig.5B. Cells were plated in methylcellulose medium containing either vehicle or 1 μM ALRN-6924 for 10 days. (D) Intracellular FACS analysis of PBMNCs isolated from the MDS/AML patient treated with ALRN-6924 at the indicated time points after infusion. Total PBMNCs were isolated and stained with hematopoietic stem cell and progenitor markers CD34 and CD38 and pan-lymphocyte markers followed by intracellular staining of p53 and p21. FACS analysis of p53 activity in AML cells was performed by gating on lymphocyte marker-negative (CD3, CD4, CD8, CD10, and CD19), CD34+CD38- cells. The MFI of p53 and p21 in CD34+CD38- cells compared to the MFI of p53 and p21 in healthy lymphocytes at the indicated times after infusion with ALRN-6924 is shown. (E) Pathological report summarizing the percentages of peripheral blood blasts at the indicated time points after infusion with ALRN-6924. (F) Peripheral blood analysis of the patient’s platelets and neutrophils at the indicated times after infusion with ALRN-6924. Error bars represent the average of technical replicates (n = 3) ± SD; *P < 0.05 unpaired one-tailed Student’s t test.
