Figure 1.
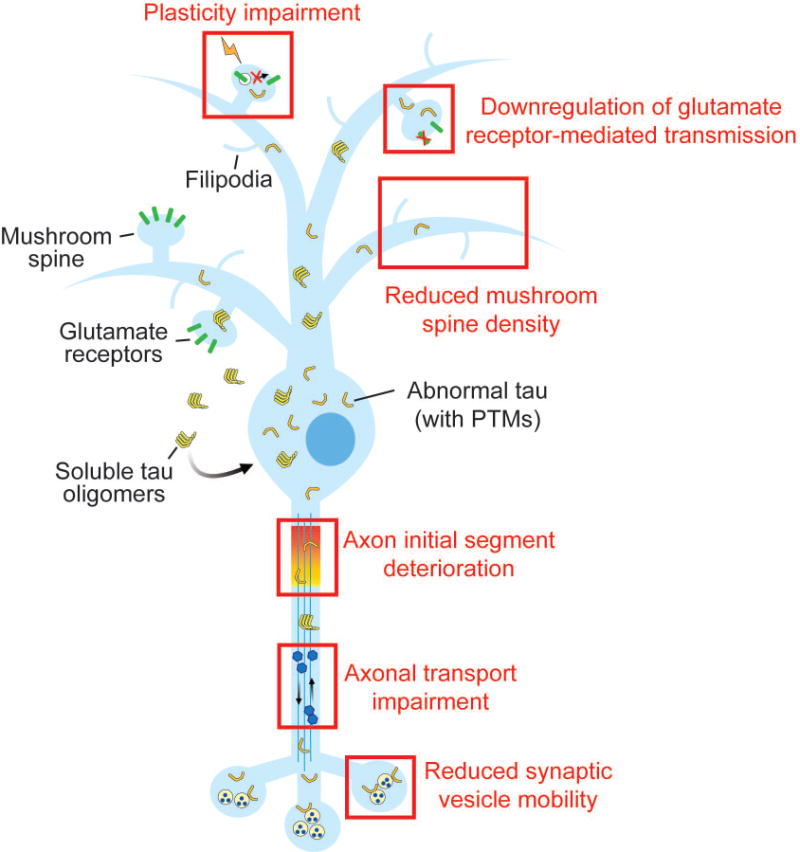
Pathogenic forms of tau, including soluble tau oligomers and tau with abnormal post-translational modifications (PTMs), can promote neuronal dysfunction by multiple mechanisms at the early stages of neurodegenerative disease. At postsynaptic sites, pathogenic tau reduces glutamate receptor levels and inhibits receptor trafficking during synaptic plasticity. The tau-mediated loss of mushroom spines is associated with increased filipodia on dendrites. Axonal function is compromised by tau due to the destabilization of the axon initial segment and deficient axonal transport. At the presynaptic terminal, tau decreases the release of neurotransmitter-containing synaptic vesicles by reducing their mobility in the terminal.
