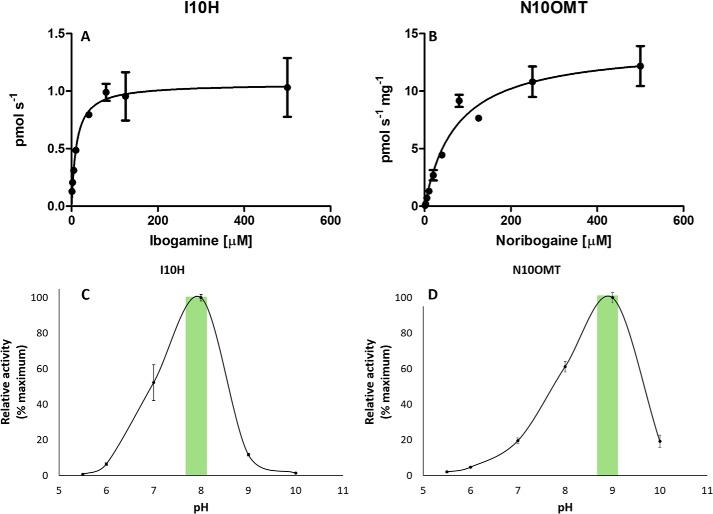
Figure 7.
Enzymatic activity of I10H and N10OMT. A and B, steady-state enzyme kinetics of recombinant I10H (A) and N10OMT (B), using ibogamine and noribogaine, respectively, as substrates. Incubation time (25 min) and protein concentration (50 μg, N10OMT) were optimized before kinetic analyses. Values represent the mean product formation (pmol s−1 I10H or pmol s−1 mg−1 N10OMT) ± S.D. (error bars) of three independent measurements. Maximum velocity (Vmax) and the concentration of substrate that permits the enzyme to achieve half Vmax (Km), catalytic rate (kcat), and catalytic efficiency (kcat/Km) were determined based on Michaelis-Menten kinetics. Shown are pH optima of I10H (C) and N10OMT (D). I10H activity was determined for the conversion of ibogamine to noribogaine. N10OMT activity is based on the conversion of noribogaine to ibogaine. C, the overall pH optimum of oxidation (I10H) and methylation (N10OMT) is indicated by the yellow highlight. Values represent the mean ± S.D. of three independent measurements.