Figure 5.
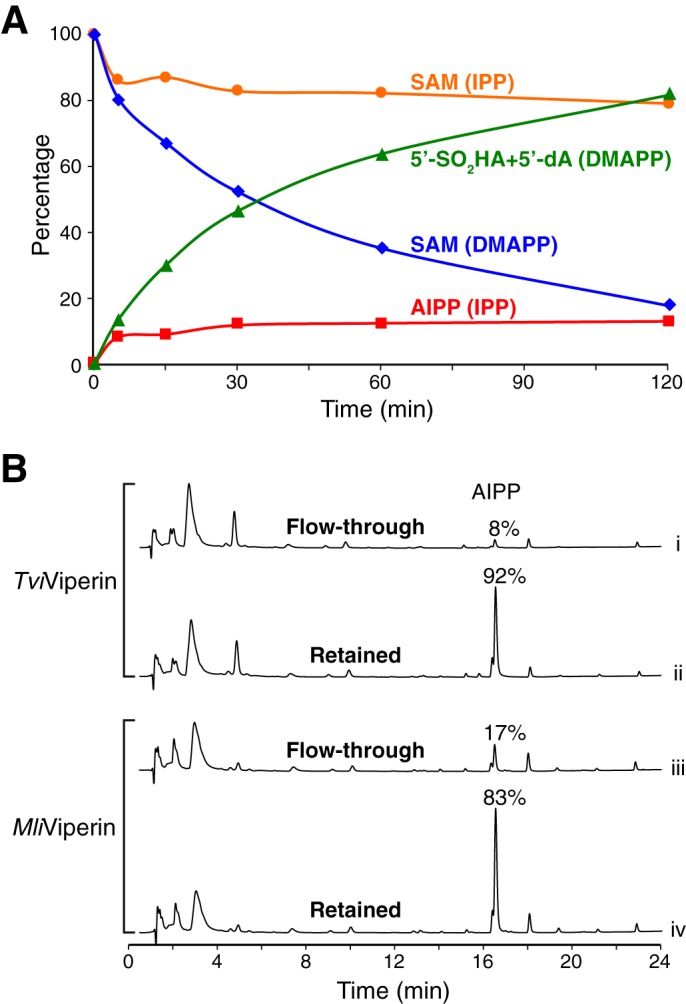
Product inhibition of the viperin-catalyzed reaction in vitro. A, time course of the TviViperin-catalyzed reaction. The concentrations of SAM and products are plotted against the reaction time for the reactions using DMAPP and IPP as substrates. Each reaction contains 50 μm TviViperin and 200 μm both SAM and the substrate. The measurement of the iron content of the purified TviViperin indicates that ∼50% protein contains [4Fe-4S]. Therefore, the molar ratio of the active enzyme and AIPP produced is ∼1:1. B, UPLC analysis of the samples from the partition experiments. Under the nondenaturing conditions, a 3-kDa centrifugal filter was used to separate small molecules free in solution (flow-through) from those bound to the enzyme (retained). An equal volume of 8 m guanidine hydrochloride was then added to both the flow-through and the retained fractions, followed by the second round of filtration. The filtrates of the second round of filtration were analyzed by UPLC.
