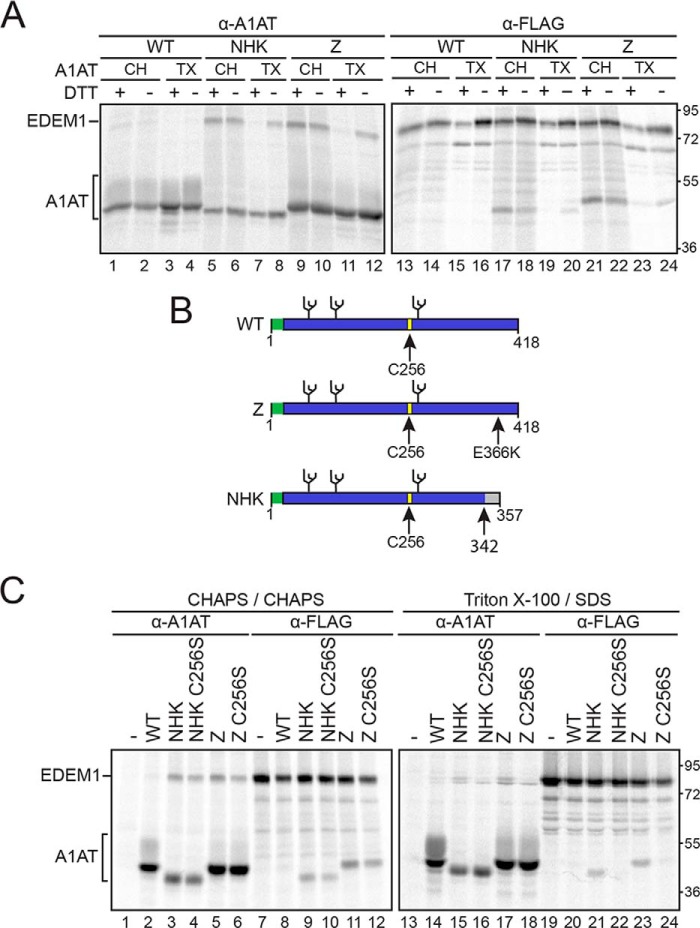
Figure 1.
EDEM1 binding to ERAD clients A1AT Z and NHK is bipartite and involves Cys256 on Z/NHK. A, EDEM1-FLAG was coexpressed with A1AT WT, NHK, or Z in HEK293T cells. Cells were radiolabeled with [35S]Cys/Met for 30 min and chased for 15 min. DTT (5 mm) was added to the cells for 30 min where indicated (+DTT). Cells were lysed in MNT buffer containing TX or CH, and EDEM1 and A1AT were isolated using anti-FLAG (α-FLAG) and anti-A1AT (α-A1AT) antisera. The proteins were resolved by 9% reducing SDS-PAGE. B, cartoon representation of A1AT constructs depicting signal peptide (green), glycosylation sites (black), and Cys256 (yellow). Arrows denote point mutation E366K on Z and frameshift mutation at 342 on NHK. C, EDEM1-FLAG was coexpressed in HEK293T cells with A1AT WT, Z, Z C256S, NHK, or NHK C256S. The proteins were radiolabeled with [35S]Cys/Met for 30 min and chased for 1 h. Cells were lysed in 2% CHAPS or MNT buffer containing 0.1% SDS. Half of the cell lysate was subjected to anti-A1AT immunoprecipitation, and the other half was subjected to anti-FLAG immunoprecipitation and washed in 0.5% CHAPS or wash buffer containing 0.1% SDS, respectively. Proteins were resolved by 9% reducing SDS-PAGE. Gels are representative of three independent experiments.