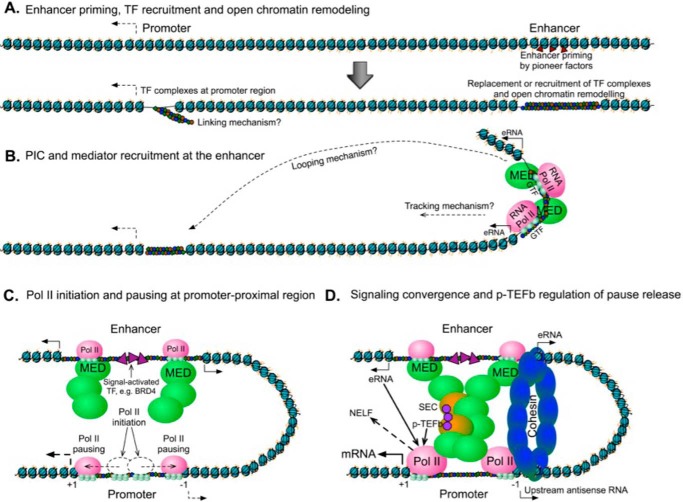
Figure 2.
Proposed steps of enhancer–promoter interaction and promoter-proximal pausing of RNAPII. A, cognate binding of pioneer factors disrupts the structure of closed, compact chromatin, together with chromatin-remodeling complexes, generating NFRs. NFRs then provide a platform for the replacement or recruitment of additional TFs. Enhancer priming by pioneer factors occurs in advance of promoter activation. The open chromatin structure resulting from enhancer priming enables the recruitment of large protein complexes to the NFRs of enhancer and promoter regions. A cascade of TF linking may occur at promoter-proximal sequences until the core promoter is bound by the recruited TFs, which provide platforms for the recruitment of PICs and Mediator. B, recruitment of large protein complexes, including the PIC and Mediator complexes occurs at the enhancer region and initiates bidirectional transcription from the enhancer. The activated enhancer may interact with the promoter via tracking or looping or a combined facilitated-tracking mechanism. The recruitment of PIC and Mediator at the promoter region may happen simultaneously with or after enhancer activation. These two situations lead to different models: 1) promoter activation may be an independent event from enhancer activation, or 2) enhancers may recruit the general transcription machinery and transfer it to the interacting promoter. C, enhancer–promoter interactions are mediated by RNAPII and the Mediator/Cohesin complex. The looped structure and RNAPII recruitment to the promoter-proximal region are associated with RNAPII pausing. RNAPII initiates transcription and progresses to the pause sites at nucleosomes flanking the promoter. Release of paused RNAPII results in divergent transcription elongation, which occurs at either the commencement of looping or thereafter. Although not shown, phosphorylation of Ser-5 in the RNAPII CTD promotes transcription initiation. In addition, subsequent to RNAPII progression to pause sites, several pausing factors, including NELF and 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) sensitivity-inducing factor, contribute to the stabilization of RNAPII pausing. D, promoter-proximal pausing of RNAPII is a rate-limiting step for productive transcriptional elongation. During signaling convergence, co-activators such as BRG4 are recruited by the TFs, resulting in the binding of elongation factors like the super-elongation complex (SEC) and Mediator. P-TEFb phosphorylates pausing factors and the CTD of RNAPII, leading to the release of paused RNAPII. Some eRNAs may facilitate the stable formation of enhancer–promoter looping through interaction with the Mediator and Cohesin complex and may also facilitate the transient release of NELF, leading to RNAPII pause release and stepping into a phase of productive elongation.