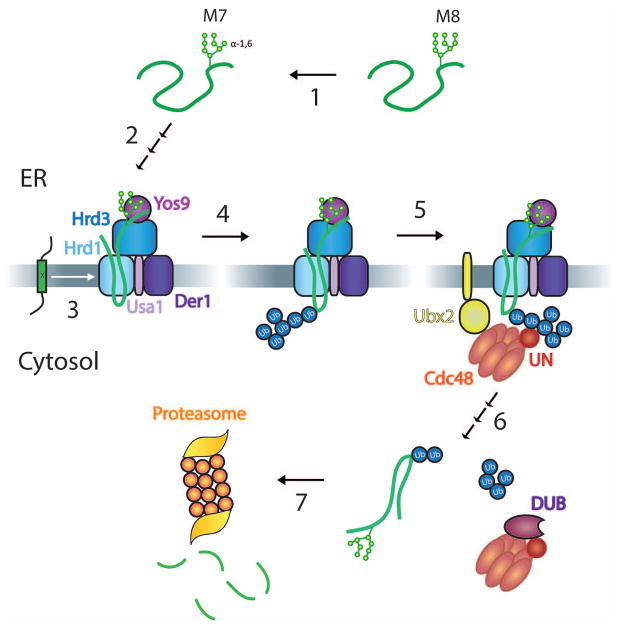
Figure 1. Overview of ERAD-L and –M.
The scheme shows different steps in ERAD-L. Step 1: The N-glycan chain of a misfolded luminal glycoprotein is trimmed from eight to seven mannoses (M8 and M7, respectively) by glycosidases. Step 2: The generated terminal α1,6-linked mannose residue binds to Yos9, and the misfolded segment around the glycan attachment site binds to Hrd3. The substrate inserts into the Hrd1 channel with the help of Der1, which associates with Hrd1 through Usa1. Step 3: ERAD-M substrates are misfolded in their membrane-spanning segments (indicated by an “x”) and enter Hrd1 sideways. Step 4: Both ERAD-L and ERAD-M substrates are polyubiquitinated by Hrd1. Step 5: The Cdc48 ATPase is recruited to the ER membrane by binding of the Ufd1/Npl4 (UN) cofactor to the ubiquitin chain and by Cdc48 binding to Ubx2. Step 6: Cdc48 uses ATP hydrolysis to pull the polypeptide substrate out of the membrane, the complex of Cdc48 ATPase and substrate leaves the membrane, and a DUB trims the ubiquitin chain, allowing release of the substrate from Cdc48. Step 7: The substrate is degraded by the proteasome.