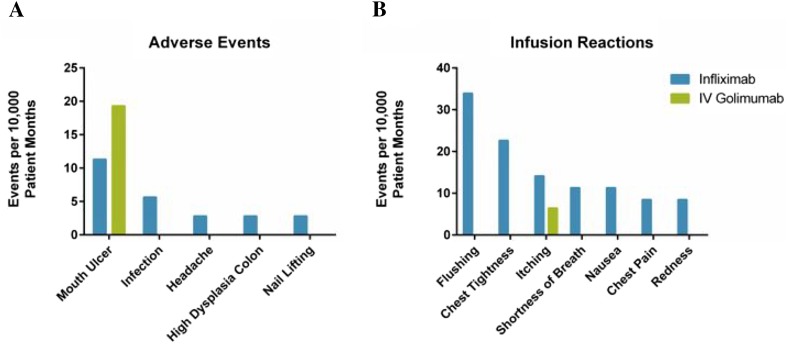
Fig. 2.
Safety events and infusion reactions. Adverse events and infusion-related reactions are presented per 10,000 patient months for patients receiving infliximab (blue) or intravenous golimumab (green). Total patient months were 3533.03 for the infliximab patient population, 1552.89 for intravenous golimumab, and 5085.92 for overall patient population. There were 25.47 adverse events and 110.38 infusion-related reactions per 10,000 patient years for patients while receiving infliximab. There were 19.31 adverse events and 6.43 infusion-related reactions per 10,000 patient years for patients while receiving intravenous golimumab. A single patient experienced all mouth ulcer-related AEs while treated with infliximab (four) and intravenous golimumab (three). In some cases, patients may have experienced more than one event while undergoing treatment