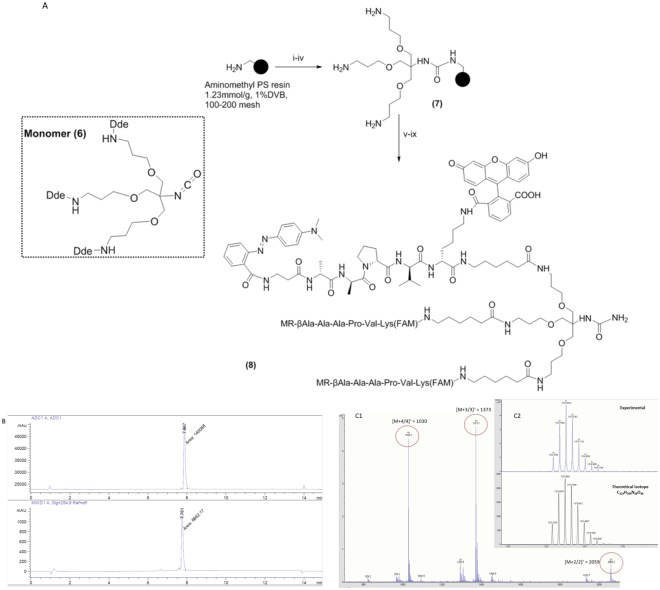
Figure 2.
(A) Probe synthesis. Reagents and conditions: (i) Fmoc-Rink amide linker, HOBt, DIC, DMF; (ii) 20% Piperidine/DMF; (iii) Monomer (6), DIPEA, DMAP, DCM/DMF; (iv) 2% Hydrazine/DMF; (v) [(a) Fmoc-AA-OH, Oxyma, DIC, DMF, (b) 20% piperidine/DMF x 6]; (vi) Methyl red, Oxyma, DIC, DMF,; (vii) (a) 2% Hydrazine/DMF, b) 5(6)-carboxyfluorescein, Oxyma, DIC, DMF; (ix) TFA/DCM/TIS (95/2.5/2.5). MR = Methyl red, FAM = 5(6)-carboxyfluorescein amide, Ahx = 6-aminohexanoic acid, Dde: N-(1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl). (B) RP-HPLC chromatogram of probe (8) on a Discovery C18 reverse-phase column (50 × 4.6 mm, 5 μm) with a flow rate of 1 mL/min and eluting with 0.1% HCOOH in H2O (A) and 0.1% HCOOH in CH3CN (B), a gradient of 5 to 95% B over 13 min and an initial isocratic period of 2 min with detection at 254 nm (lower) and by evaporative light scattering (upper); (C) FTMS analysis: m/z 1030 [M + 4/4]+, 1373 [M + 3/3]+ and 2059 [M + 2/2]+; insert spectral zoom (experimental and theoretical [M + 3/3]+).