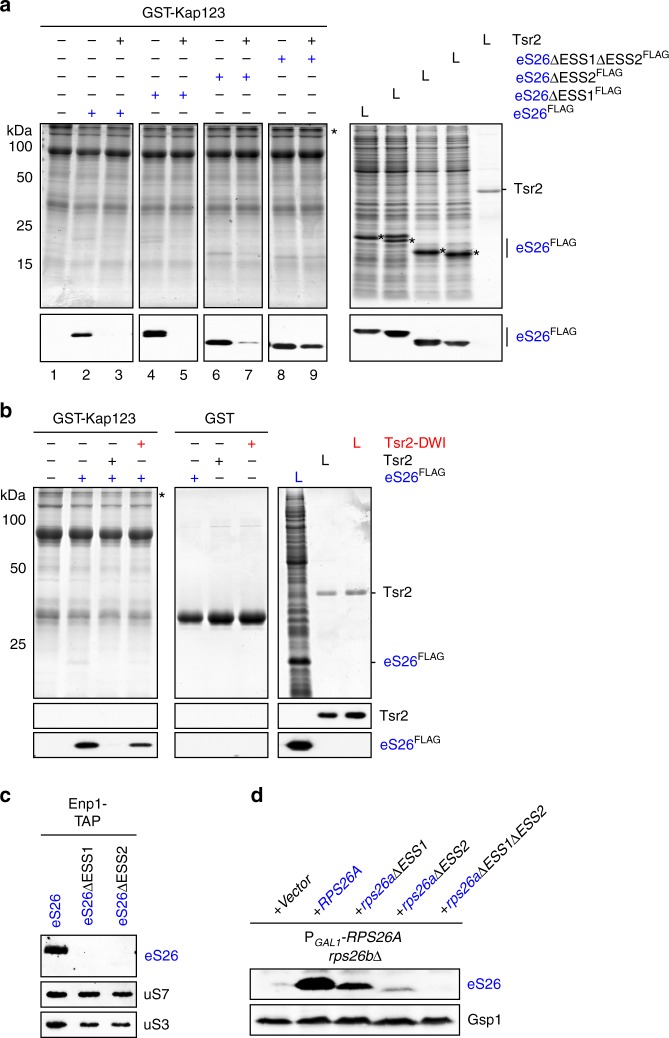
Fig. 4.
ESSs mediate Tsr2-dependent importin:eS26 complex disassembly. a Tsr2 cannot efficiently dissociate the Kap123:eS26ΔESS1ΔESS2FLAG complex. The complex of GST-Kap123 with eS26FLAG, eS26ΔESS1FLAG eS26ΔESS2FLAG or eS26ΔESS1ΔESS2FLAG was immobilized on Glutathione Sepharose and incubated with either buffer alone or with Tsr2 before pull-down. L = input (1:10 diluted). b Tsr2DWI mutant does not dissociate a Kap123:eS26 complex. The complex of GST-Kap123 with eS26FLAG was incubated with either buffer alone, purified Tsr2 or Tsr2DWI before pull-down. L = input (1:10 diluted). c Efficient recruitment of eS26 to pre-40S requires both of the ESSs. Enp1-TAP was isolated from PGAL1-RPS26Arps26b∆ strain transformed with WT eS26 or eS26 lacking ESS1 or ESS2. After tandem affinity purification, eluates were separated by 4–12% gradient SDS-PAGE and subjected to western analyses using indicated antibodies. Protein levels of uS7, uS3 served as a loading control. d Protein levels of eS26, eS26ΔESS1, eS26ΔESS2 and eS26ΔESS1ΔESS2 in whole-cell extracts of PGAL1-RPS26Arps26b∆ strain were determined by western analyses using α-eS26 antibodies. Gsp1 protein levels served as a loading control