Table 2.
Structures, receptor affinities, and actions of anti-emetic drugs.
| D1 | D2 | D3 | D4 | α1 | α2 | H1 | M | 5-HT2A | 5-HT3A | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic Receptor Antagonist | |||||||||||
Scopolamine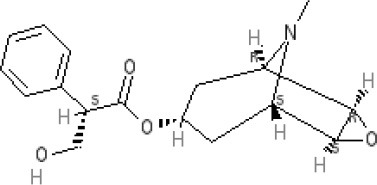
|
Ki >10,000 nM (rat)13 | Ki >10,000 nM (rat)13 | M1 9.0 M2 8.7 M3 9.4 M4 9.5 antagonist | ||||||||
| Histamine H1 Receptor Antagonists | |||||||||||
Diphenhydramine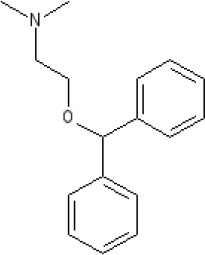
|
Ki >10,000 nM (rat)13 | 7.9 antagonist | pA2 7.1 antagonist (rat)7 | ||||||||
Promethazine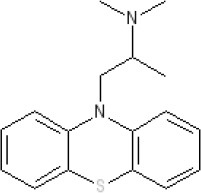
|
Ki 240 nM (rat)13 | 9.6 antagonist | Ki 21 nM (rat)13 | ||||||||
Cyclizine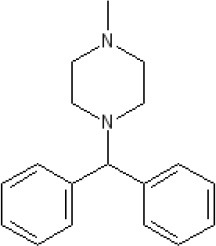
|
8.4 antagonist | Pre-ganglionic cholinergic inhibition (animals)15 | |||||||||
Cinnarizine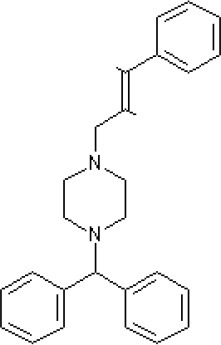
|
antagonist16 | antagonist16 | Blocks L-type and T-type calcium channels16 | ||||||||
| Phenothiazines | |||||||||||
Prochlorperazine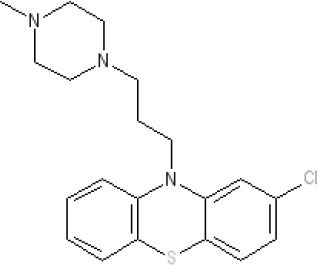
|
7.1 antagonist | 8.4 antagonist | 8.4 antagonist | 6.1 antagonist | Ki 200 nM (rat)14 | p1C50 6.7 inverse agonist6 8.28 | Ki 2,100 nM (rat)13 | 8.23 | Inactive (rat)10 | ||
Chlorpromazine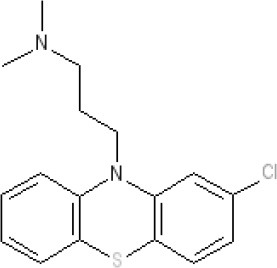
|
7.1 antagonist | 7.0–7.6 antagonist | 7.2–7.5 antagonist | 7.8 antagonist | α1A Ki 0.28 nM12 | α2A 5.9–6.6 α2B 7.2–8.3 α2C 6.9–7.4 antagonist at each | 8.2 antagonist | Ki 47 M3 (rat)12 | 8.1 inverse agonist | Inactive (rat)10 | D5 6.9 antagonist 5-HT1A 6.2 antagonist 5-HT2C 7.6–8.2 antagonist 5-HT6 7.7–7.8 inverse agonist 5-HT7 7.6 inverse agonist |
Fluphenazine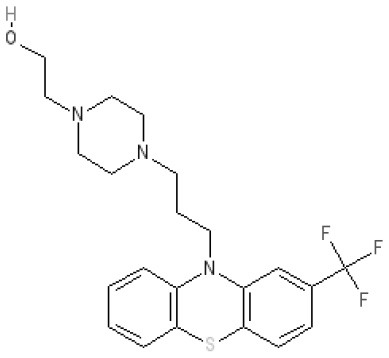
|
7.7 antagonist | 8.8 antagonist | Ki 8.1 nM (rat)14 | 7.7 antagonist | Ki 340nM (rat)13 | 7.5 antagonist | Inactive (rat)10 | D5 7.9 antagonist 5-HT7 7.9 inverse agonist 5-HT6 7.3–7.4 inverse agonist | |||
Levomepromazine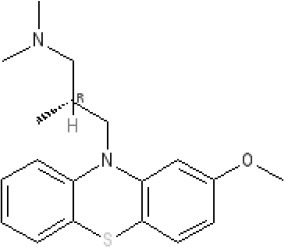
|
Ki 54.31 | Ki8.61 | Ki8.31 | Kd 0.58nM antagonist2 | |||||||
Mirtazapine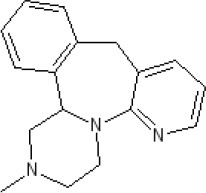
|
α2A 7.7 antagonist | α2C 7.7 antagonist | p1C50 9.6 inverse agonist68.98 | 7.2 antagonist | 5-HT2C 7.4 antagonist | ||||||
| Metoclopramide | |||||||||||
Metoclopramide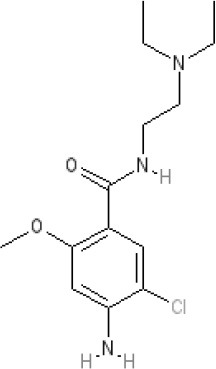
|
7.5 antagonist (mouse) | Ki >10,000 nM (rat) | Ki 1,100 nM (rat)13 | Ki >10,000 nM (rat)13 | 5-HT3A 6.0–6.4 antagonist 5-HT3AB 5.7 antagonist | 5-HT4 6 agonist (mouse) | |||||
| Butyrophenones | |||||||||||
Haloperidol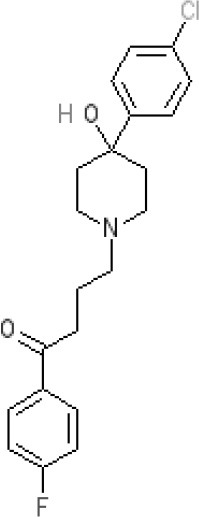
|
7.6–8.2 antagonist | 7.4–8.8 antagonist | 7.5–8.6 antagonist | 8.7–8.8 antagonist | Ki 46 nM5 | Ki 360 nM5 | 5.7–6.1 antagonist | Ki >1,000 nM at M1, M2, M3 (rat)5 | 6.7–7.3 antagonist | Ki >1,000 nM5 | 5-HT1D 6.6 antagonist 5-HT7 6.3–6.6 antagonist 5-HT2B 5.8–6.4 antagonist 5-HT1A 5.7–5.8 antagonist |
Droperidol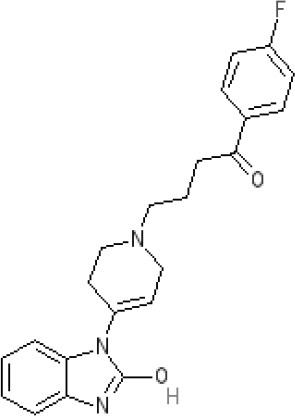
|
Ki 3 nM (rat)11 | Ki 1.4 nM (rat)11 | Ki 2,500 nM (rat)11 | Ki 4.6 nM (rat)11 | Inactive (rat)10 | ||||||
Domperidone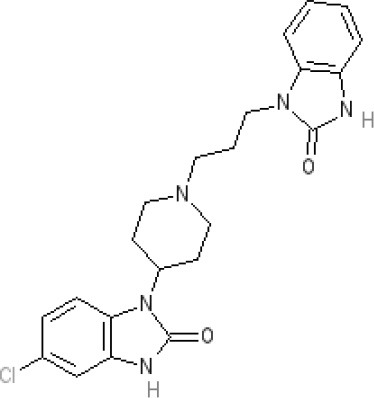
|
7.9–8.4 antagonist | 7.1–7.6 antagonist | Ki 30.4 nM4 | Ki α1A, 71; α1B, 530; α1D, 710 nM9 | Inactive (rat)10 | ||||||
| Second generation anti-psychotics | |||||||||||
Olanzapine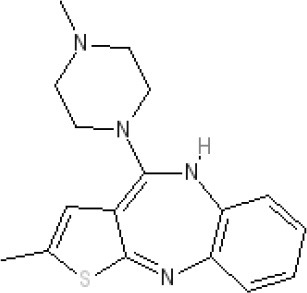
|
Ki 31 nM (rat)5 | 8.7 antagonist | Ki 27 nM (rat)5 | α1A Ki 115 nM12 | α2A Ki 314; α2B 81.6; α2C 28.8 nM12 | 8.7–9.2 antagonist | Ki 105 nM at | 8.6–8.9 antagonist | Ki 57 nM (rat)5 | 5-HT2C 8.1–8.4 inverse agonist 5-HT6 8 inverse agonist 5-HT7 6.5 antagonist | |
| 5-HT3 Receptor Antagonists | |||||||||||
Granisetron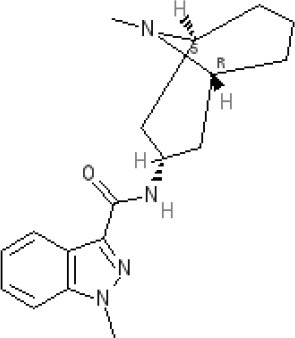
|
Inactive (>10,000 nM) (rat)10 | 5-HT3A ~8.6–8.8 antagonist | |||||||||
Ondansetron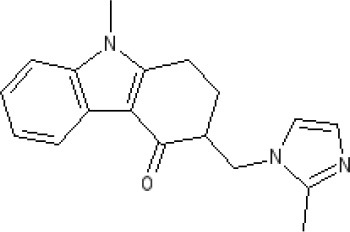
|
5-HT3A ~7.8–8.3 5-HT3AB 7.8 antagonist | ||||||||||
Tropisetron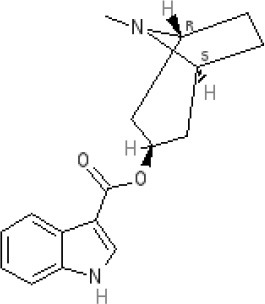
|
Inactive (>10,000 nM) (rat)10 | 5-HT3A 8.5–8.8 antagonist | 5-HT4 6.3–7.1 antagonist | ||||||||
Palonsetron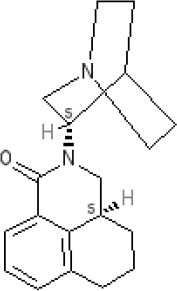
|
5-HT3A 10.5 antagonist | ||||||||||
| NK1 receptor antagonists | |||||||||||
Aprepitant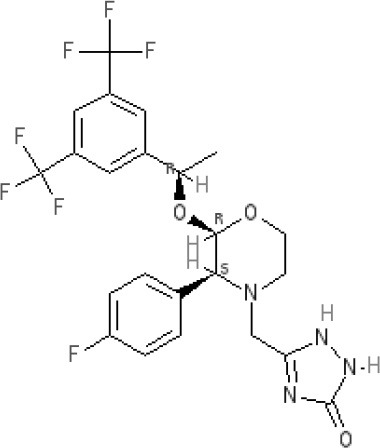
|
NK1 10.1 antagonist | ||||||||||
Data are from http://www.guidetopharmacology.org/ and are given as pKi-values for the human receptors (usually recombinant); primary affinities are in bold. Additional information on drug affinity for human receptors (unless otherwise specified) is provided in italics together with references, listed below. Little information is known for cyclizine or for cinnarizine, particularly in terms of their interactions with human receptors and mechanisms. Clarke and Dundee (1971) describe significant sedation/drowsiness associated with promethazine, thiethylperazine, cyclizine and hyoscine. 1Srivastava et al., 2009; 2Kanba and Richelson, 1984; 3Meltzer et al., 1989; 4Moreland et al., 2004; 5Bymaster et al., 1996; 6Bakker et al., 2007; 7Liu et al., 2006; 8Appl et al., 2012; 9Keiser et al., 2009; 10Hamik and Peroutka, 1989; 11Peroutka and Synder, 1980; 12Kroeze et al., 2003; 13Peroutka and Snyder, 1982; 14Ison and Peroutka, 1986; 15Norton et al., 1954; 16https://www.drugbank.ca/drugs/DB00568#BE0000442.
