Extended Data Figure 1. Biochemistry, binding, electrophysiology and α4β2-Fab interactions.
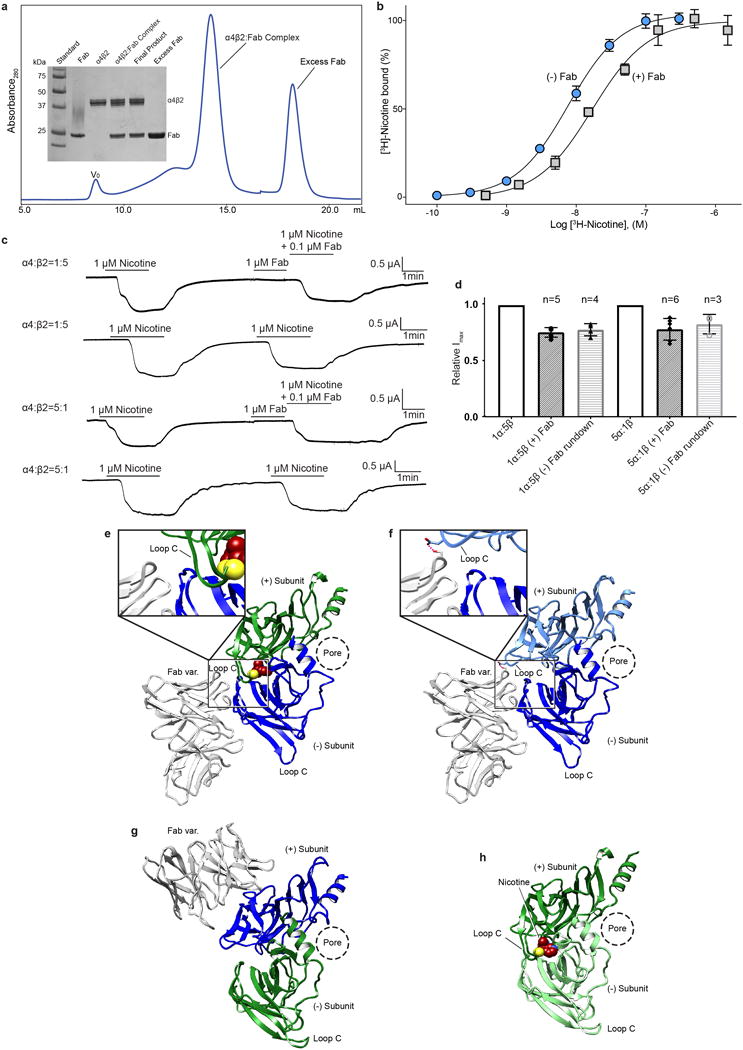
a, Size-exclusion chromatogram of α4β2:Fab complex and SDS-PAGE analysis of complex purification (V0, void volume). b, Saturation binding experiments with [3H]-nicotine and a mixture of receptor subunit stoichiometries. Grey squares denote samples with receptor plus Fab and blue circles denote samples with receptor alone. Receptor alone Kd = 7.7 nM (95% confidence interval of 6.9-8.6 nM) and receptor plus Fab Kd = 17.0 nM (95% confidence interval of 10.2-26.6 nM). Receptor alone and receptor plus Fab both exhibited a hill slope of ~1 (1.14 and 1.09 respectively). Plotted results are from a representative experiment performed in triplicate. c, Representative two-electrode voltage clamp (−60 mV) recordings of oocytes injected with cRNAs for the α4 and β2 subunits in ratios to bias assembly,1α:5β (top two traces) and 5α:1β (bottom two traces) for the 2α:3β and 3α:2β assemblies respectively. The experiments were performed in the presence and absence of Fab to assess effect of Fab on receptor gating. Each oocyte was perfused with a 1 μM nicotine solution for ~2 min, washed with bath solution for 10 min and then perfused for ~2 min with 1 μM nicotine +/− Fab. For samples were Fab was included, 1 μM final [Fab] was added directly to the oocyte bath following the first perfusion and allowed to incubate for 1.5 min before perfusing with a second solution containing 1 μM nicotine + 0.1 μM Fab. d, Bar graph quantifying peak currents before and after adding Fab. Currents were normalized to the amplitude of the first nicotine application. Appreciable current rundown was observed (assessed by applying nicotine without Fab as the second application). Change in peak current is similar in the presence and absence of Fab for both stiochiometries, suggesting no substantial effect on gating. n values are number of oocytes; error bars are standard deviation from the mean. e-h, Fab:β subunit interactions. With one exception, the Fab molecules interact exclusively with a single β subunit. The exception is at the β-β interface, where the Fab on the complementary β subunit forms one potential interaction with the preceding β subunit. This interaction is displayed as a dashed line in the panel f inset. The conformation of the principal Loop C at the exceptional β-β interface is indistinguishable from those where Fab is not interacting, suggesting that Fab does not affect the loop C conformation. e-h, Fab interactions at the α-β, β-β, α-β, and α-α interfaces, respectively. Subunits are colored as in Fig. 1.
