Extended Data Figure 11. Comparison of putative cholesterol binding orientations.
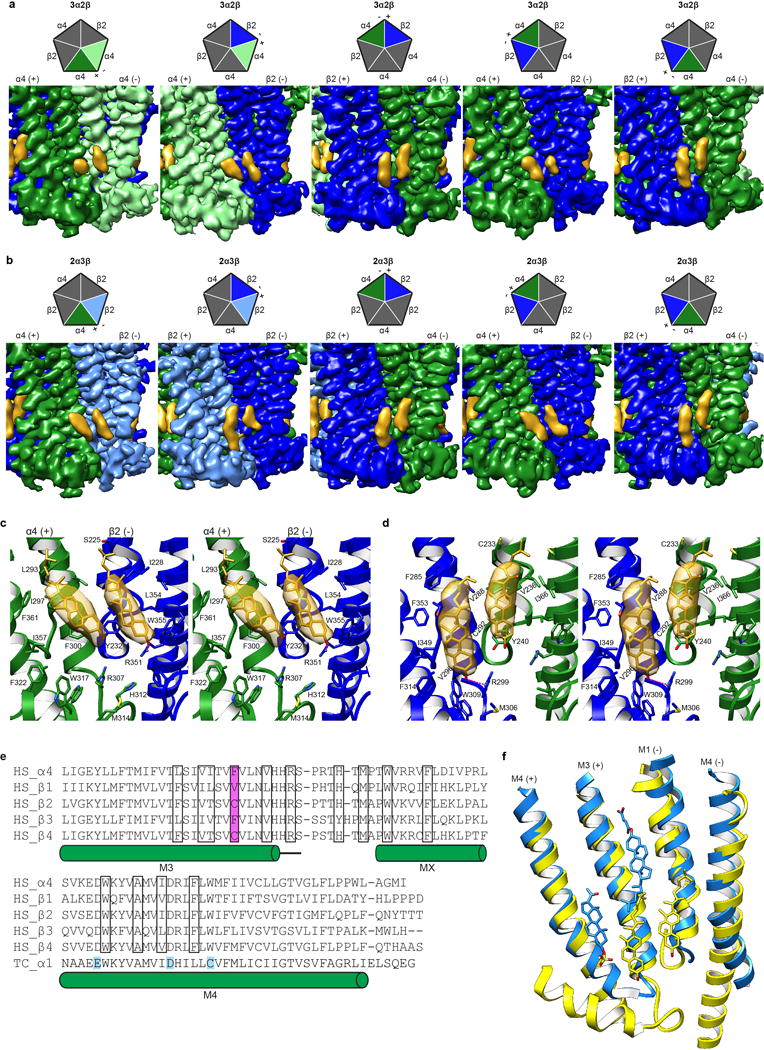
a-b, EM density map showing cholesterol sites from all TMD interfaces in the 3α:2β assembly and 2α:3β assembly respectively. Cartoon pentagons (top) are colored to illustrate subunits composing the displayed interface. c, Stereo image of Representative cholesterol binding site with α on principal side (+). When the principal side (+) is an α4 subunit both molecules tilt toward the principal face. One residue from the complementary side β2 (−), Y232, contacts both cholesterol molecules at the α-β interface (at the α-α interface the equivalent residue Y240 in the complementary (−) α4 contacts both cholesterol molecules at the interface; not shown). d, Stereo image of Representative cholesterol binding site with β on principal side (+). When the principal side (+) is a β2 subunit both cholesterol molecules are oriented orthogonal to the plane of the membrane. One residue from the principal (+) β2 subunit, V288, makes contacts with both cholesterol molecules at the β-α and the β-β (not shown) interface. Cholesterol and interacting side chains are shown as sticks. Density maps in panels a-d are displayed at a threshold of 0.025 in Chimera. e, Alignment of nicotinic receptor subunits for region encompassing putative cholesterol sites. Residues in cholesterol binding pocket are boxed and amino acid position implicated in differential cholesterol binding is highlighted in magenta. Uniprot accession IDs are provided40. Residues mapped using a photoreactive cholesterol analog in Torpedo californica (TC P02710)26,27 are highlighted in cyan. All other sequences are from Homo sapiens (HS P43681, P11230, P17787, Q05901, P30926 for α4 and β1-4 respectively). f, Superposition of β-α (yellow) from α4β2 and α1-α1 (Dodger blue) GABAA-GLIC chimera (PDB accession 5OSC25) TMD interface to compare putative binding sites. Pregnenolone sulfate, CHS and cholesterol are shown as sticks.
