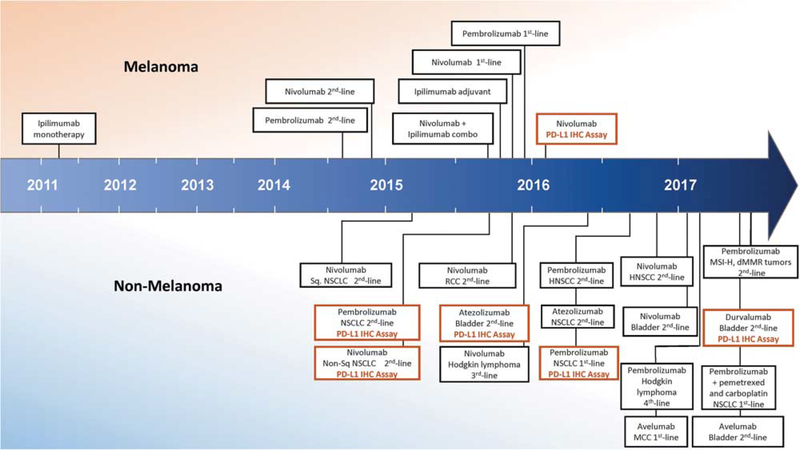
Figure 3.
Timeline of FDA approvals for immune checkpoint blocking agents, including PD-L1 immunohistochemistry companion and complementary diagnostics. The earliest approvals were provided for patients with melanoma, including in 2011 for ipilimumab (antiCTLA-4), in 2014 for nivolumab and pembrolizumab (anti-PD-1 agents) monotherapy, and in 2015 for combined ipilimumab and nivolumab. Anti-PD-1/PD-L1 agents for other tumor types first received approval in 2015, and the number of indications is rapidly expanding. Companion and complimentary PD-L1 immunohistochemistry (IHC) diagnostics were first approved in 2015 for patients with non-small cell lung carcinoma (NSCLC) in the second-line setting. In late 2016, however, pembrolizumab (anti-PD-1) and the companion PD-L1 IHC diagnostic secured approval as a first-line treatment for NSCLC. (Abbreviations: combo, combination; dMMR, mismatch repair deficient; HNSCC, head and neck squamous cell carcinoma; MCC, Merkel cell carcinoma; MSI-H, microsatellite instability-high; RCC, renal cell carcinoma; Sq, squamous).