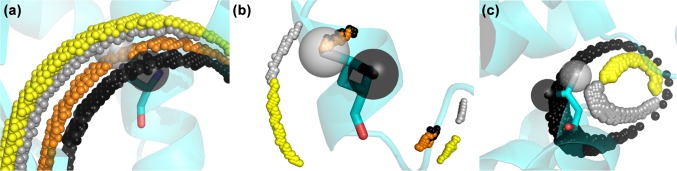
Fig. 9.
Positions for methyl groups in the ntd-HSP90 bound to 1. Small spheres represent PCS iso-surfaces cross-sections in the free state, grey for Valγ1/Leuδ1 and black for Valγ2/Leuδ2, and in the bound state, yellow for Valγ1/Leuδ1 and orange for Valγ2/Leuδ2. The residues as found in the crystal structure of the free protein (PDB entry 3t0h) are shown in cyan sticks, with the methyl groups in the analogous colors shown as 0.7 Å radius spheres centred on the carbon methyl. a Leu48, with cross-sections for PCS from mutants 50C/54C and 101C/105C. The minimal distance from the crystal structure conformation to the free cross-sections is 0.8 Å (δ1) and 0.6 Å (δ2), and to the bound cross-sections is 1.3 Å (δ1) and 1.2 Å (δ2). b Leu103, the yellow and orange spheres represent the triple cross-sections of mutant 50C/54C with 101C/105C and 149C/187C. The grey and black spheres represent the triple-mutant cross-sections for the free state, at a distance of 1.6 Å (δ1) and 2.3 Å (δ2) from the crystal structure positions. Note that the orange and black region overlap whereas the grey and yellow are bordering, suggesting that methyl δ1 moves, whereas δ2 does not. c Val136, the grey and yellow small spheres represent the triple cross-section for methyl group γ1 in free and bound state respectively. The small black spheres represent the free double cross-section between mutant 50C/54C and 101C/105C of group γ2. The orange cross-section is not shown because the γ2 methyl group showed insignificant PCS changes