Fig. 2.
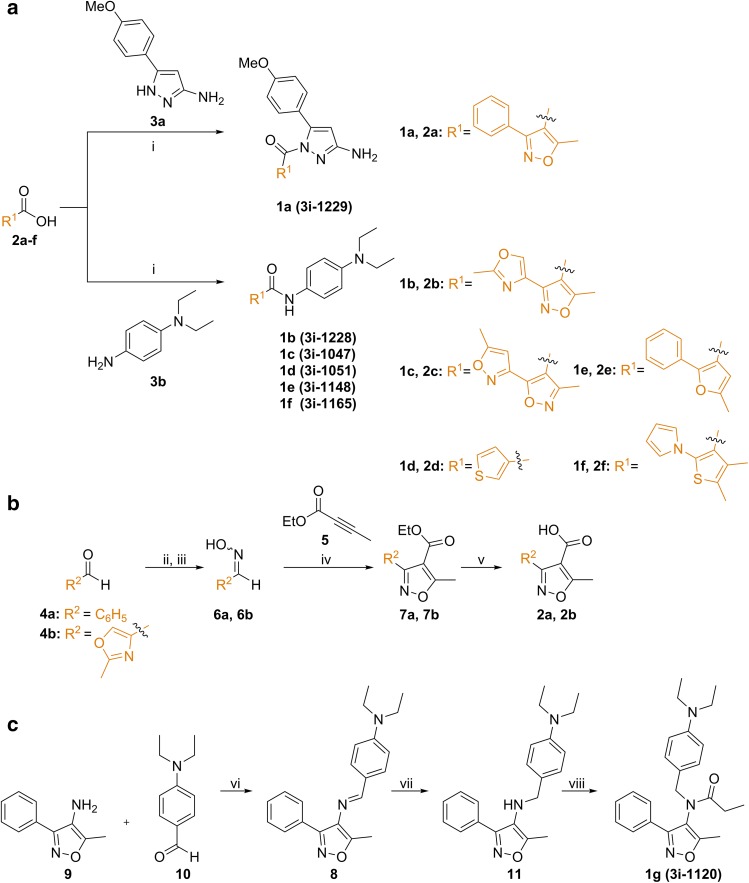
Synthesis of compounds. a Synthesis of amides. Reagents and conditions: (i) HBTU, DIPEA, DMF, rt, 1 d, 49–73%. b Synthesis of the carboxylic acid intermediates. Reagents and conditions: (ii) H2NOH·HCl, pyridine, EtOH; (iii) H2NOH·HCl, NaOAc; (iv) Oxone®, KCl, H2O; (v) HTIB, H2O. c Synthesis of the N,N-disubstituted amide via imine formation. Reagents and conditions: (vi) AcOH, Na2SO4 (anhydr.) rt, overnight, 74%; (vii) NaBH4, MeOH, THF, rt, 2 d, 37%; (viii) EtCOCl, DMAP, pyridine, rt, 3 d, 74%