Fig. 5.
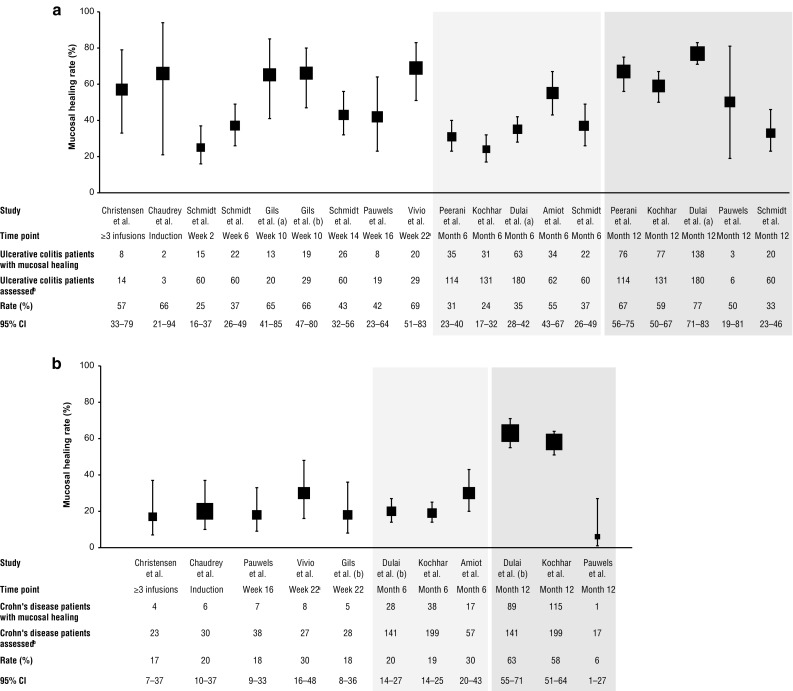
Mucosal healing rates among patients with ulcerative colitis (a) or Crohn’s disease (b) receiving vedolizumab. Square size represents the weight given to each study, based on sample size. Error bars represent 95% CIs. CI confidence interval. Christensen et al. [69], Chaudrey et al. [26], Schmidt et al. [103], Gils et al. (a) [47], Gils et al. (b) [77], Pauwels et al. [34], Vivio et al. [40], Peerani et al. [35], Kochhar et al. [83], Dulai et al. (a) [28], Amiot et al. [23], Dulai et al. (b) [46]. aMedian time point. bOnly patients with ≥ 1 follow-up assessment at the specified time point were included in the analyses. Data from the VICTORY Consortium, which contributed the majority of mucosal healing data, used a cumulative incidence analysis, and remaining studies employed a “complete” case approach
