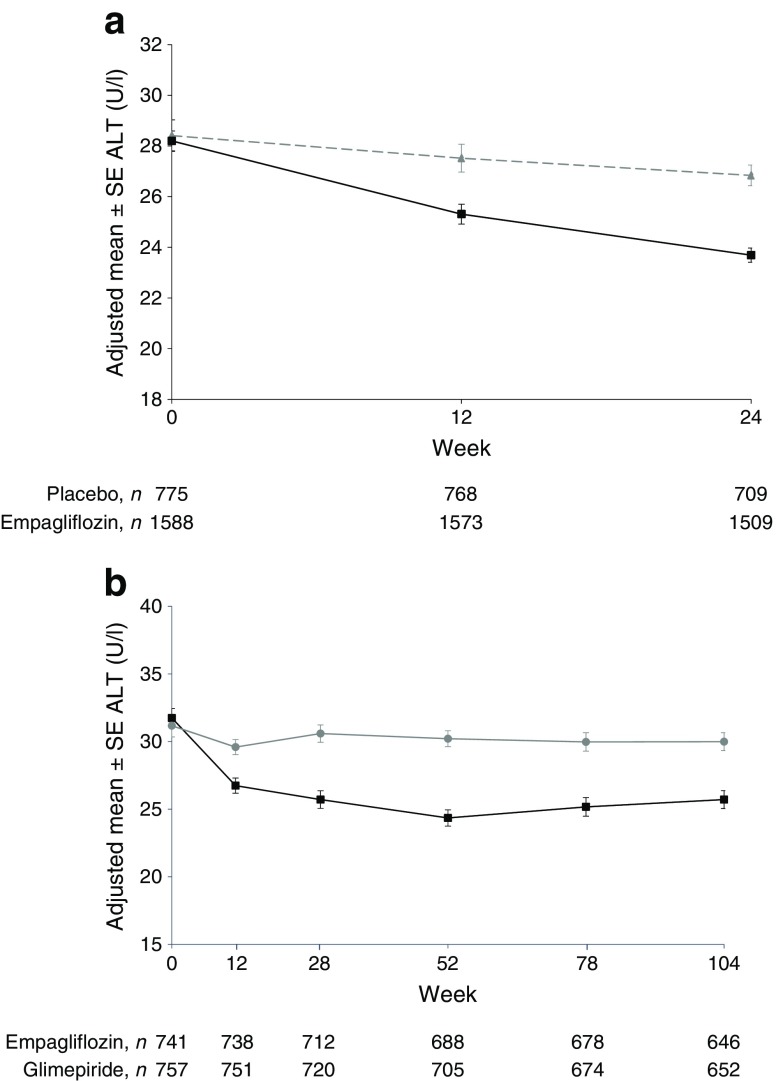
Fig. 2.
(a) Changes in ALT in pooled 24 week trial data. Adjusted mean ± SE change from baseline at week 24: −1.40 ± 0.41 with placebo and −4.55 ± 0.28 with empagliflozin (grey triangles and dashed line, placebo; black squares and solid line, empagliflozin [25 mg]). (b) Changes in ALT in the EMPA-REG H2H-SU trial. Adjusted mean ± SE change from baseline: −0.77 ± 0.64 with glimepiride and −5.65 ± 0.65 with empagliflozin at week 28; −1.37 ± 0.66 with glimepiride and −5.65 ± 0.66 with empagliflozin at week 104 (black squares and line, empagliflozin [25 mg]; grey circles and line, glimepiride). MMRM analysis in patients treated with at least one dose of study drug based on observed cases, including values after initiation of rescue medication. Baseline values are mean ± SE