FIGURE 1.
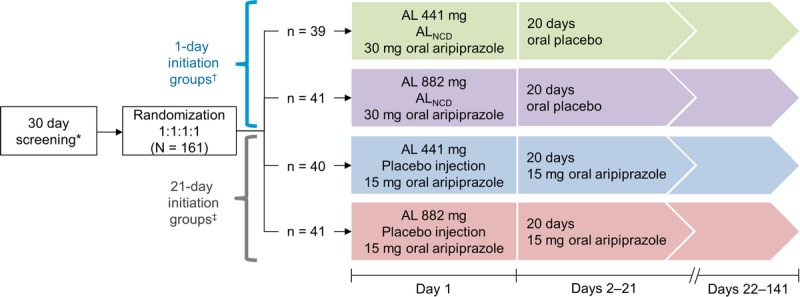
Study design. Patients were admitted to an inpatient facility on day −1 and were discharged after assessments on day 15. After discharge, patients received outpatient follow-up assessments until day 141. *Patients naive to aripiprazole were administered 5-mg test doses of oral aripiprazole on day −30 and day −29. †Patients in the 1-day initiation groups were treated as follows: ALNCD intramuscular (gluteal) plus 30-mg oral aripiprazole plus either intramuscular AL 441 mg (deltoid) or AL 882 mg (contralateral gluteal) on day 1 followed by 20 days of oral placebo. ‡Patients in the 21-day initiation groups were treated as follows: placebo injection (gluteal) plus 15-mg oral aripiprazole plus either intramuscular AL 441 mg (deltoid) or AL 882 mg (contralateral gluteal) on day 1 followed by 20 days of 15-mg oral aripiprazole.
