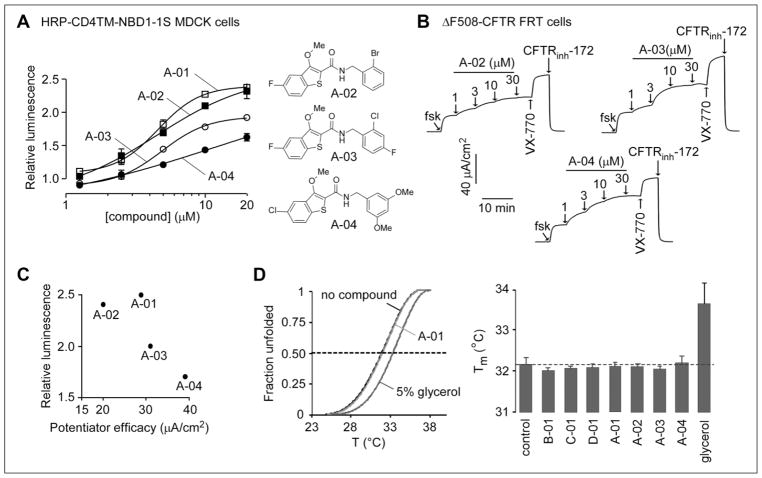
Figure 4.
Structure–activity analysis of carboxamide-benzothiophene A-01 analogs. (A, left) Concentration dependence of compound action in MDCK cells expressing HRP-ΔF508-NBD1. Compounds were incubated with MDCK cells for 24 h at 37 °C prior to assay of HRP activity (mean ± SEM; n = 3). (A, right) Structures of active analogs. (B) Compound potentiator activity measured by short-circuit current in FRT cells expressing full-length ΔF508-CFTR showing responses to forskolin (20 μM) and indicated concentrations of compounds. Cells were incubated at 27 °C for 24 h before measurement. VX-770 (1 μM) and CFTRinh-172 (10 μM) were added as indicated. (C) Plot comparing biochemical (normalized relative luminescence, y axis) and functional (x axis) ΔF508-CFTR potentiator data for the four active carboxamide-benzothiophenes. (D) Representative melting curves (left) and melting temperature (right, n = 2) of human ΔF508-NBD1-1S determined by differential scanning fluorometry. The indicated compounds (30 μM) or the chemical chaperone glycerol (5%) was present during thermal unfolding.