Abstract
HHV-6 is a member of the β-herpesvirinae subfamily. Most people acquire HHV-6 primary infection early in life and reactivation may occur, most often in immunocompromised individuals, leading to various clinical manifestations. HHV-6 infected cells may be identified in lymph nodes in both reactive and neoplastic conditions. Cases were retrieved from the hematopathology consultation service archives at National Institutes of Health from 2003 to 2017 in which infection by HHV-6 had been documented by immunohistochemical stains to HHV-6 gp60/110 envelope glycoprotein. Five cases of reactive lymphadenitis and 3 cases of lymphoma; 2 angioimmunoblastic T cell lymphoma and 1 classical Hodgkin lymphoma, positive for HHV-6 were identified. The reactive lymph nodes showed marked paracortical hyperplasia and admixed large atypical lymphoid cells exhibiting pleomorphic nuclei, vesicular chromatin, and prominent eosinophilic intranuclear inclusions. Vascular proliferation and necrosis were also present, raising suspicion of peripheral T-cell lymphoma. The three cases of lymphoma showed similar viral inclusions, in addition to the characteristic features diagnostic of the lymphoma. Staining for HHV-6 was positive with a membranous and Golgi pattern and was restricted to cells with evident inclusions on H&E. HHV-6 infected cells were positive for CD3 and CD4. HHV-6 lymphadenitis can present with morphologic atypia creating a diagnostic pitfall for lymphoma. In such cases, careful attention to the characteristic viral inclusions can lead to immunohistochemical analysis highlighting the replicating virus. In cases of lymphoma, identification of the inclusions is key in detecting the associated infection as well as in avoiding misinterpretation of the lymphoma subtype.
Keywords: Human herpes virus–6, acute lymphadenitis, lymphoproliferative disorder, viral inclusions, peripheral T-cell lymphoma
Introduction
Human herpes virus – 6 (HHV-6) was first isolated from patients with acquired immunodeficiency syndrome and lymphoproliferative disorders and was named Human B lymphotropic virus (HBLV)(1). Later, it has been shown that this virus is the causative agent of exanthem subitum in children (2). The name HHV-6 was first proposed by Ablashi et al (3). Subsequently, HHV-6 has been shown to be associated with hepatitis (4), encephalitis (5), mononucleosis-like syndrome (4, 6) and acute lymphadenitis in immunocompetent adults (7, 8). HHV-6 infection has also been reported in association with angioimmunoblastic T cell lymphoma (AITL), (9) adult T cell leukemia and B cell lymphomas (1).
HHV-6 is a member of the subfamily β-herpesvirinae, which also includes cytomegalovirus (CMV) and HHV-7. There are two distinct species identified; HHV-6A and HHV-6B based on the distinct epidemiology, disease associations, and biological and immunological properties (10). These two viruses have co-linear genomes which share 90% overall identity (11). In industrialized countries, most infections are caused by HHV-6B (12, 13) and HHV-6A is the predominant cause of childhood viremic infection in Sub-Saharan Africa (14). The prevalence of infection in the general population is high as shown by serologic and PCR based methods. The infection is acquired early in life and the virus remains latent in the host cells but may get reactivated later in life (15, 16).
Morphologic features of HHV-6 associated lymphadenitis have been described by various groups (7, 8) and it has been described in association with lymphoproliferative disorders (1, 9, 17). However, past reports have not emphasized the key diagnostic features of HHV-6 infection in benign lymphadenitis as distinct from lymphoma.
We describe 5 cases of viral lymphadenitis due to HHV-6 infection, 2 cases of AITL and 1 case of classic Hodgkin lymphoma (CHL) associated with HHV-6 infection with an emphasis on diagnostic pitfalls in histopathologic examination.
Material and Methods
Cases were retrieved from the archives of hematopathology consultation service at National Cancer Institute/National Institutes of Health from 2003 to 2017 in which HHV-6 viral infection was identified by morphology and then confirmed by immunohistochemical stain using an antibody against HHV-6 gp60/110 envelope glycoprotein, which identifies both HHV-6 A and B subtypes (Biodesign Inter, Saco ME, USA). Two of the cases of reactive lymphadenitis (case 6 and 7, see Table 1) were previously reported (7). In addition, 20 cases of AITL, without morphological evidence of viral inclusions were tested by immunohistochemistry for presence of HHV-6. No cases of reactive lymphadenitis without morphologically identifiable inclusions were tested by immunohistochemical stains.
Table 1.
Details of the demographics, clinic-pathological findings and molecular findings of the 8 cases with HHV-6 positivity.
| Case | Age | Gender | Clinical Presentation | Lymph node | Immune status | EBV | Diagnosis | Viral inclusions | Type of cells with inclusions | TRG |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | Generalized lymphadenopathy, B symptoms | Left inguinal | No known compromise other than lymphoma | Positive | AITL | Yes | CD4 T | Clonal |
| 2 | 60 | M | Generalized lymphadenopathy, B symptoms, skin rash | Right cervical | No known compromise other than lymphoma | Positive | AITL | Yes | CD4 T | Clonal |
| 3 | 17 | M | Generalized lymphadenopathy, lung nodules, B symptoms | Supraclavicular | No known compromise other than lymphoma | Positive in HRS cells | CHL | Yes | CD4 T | Not done |
| 4 | 64 | M | Generalized lymphadenopathy | Right axillary | No known compromise | Negative | Lymphadenitis | Yes | CD4 T | No amplification |
| 5 | 53 | M | Fever, Generalized lymphadenopathy | Not specified | No known compromise | Not done | Lymphadenitis | Yes | T | Not done |
| 6* | 28 | M | Fever, Generalized lymphadenopathy | Cervical | No known compromise | Negative | Atypical paracortical hyperplasia | Yes | CD4 T | Polyclonal |
| 7* | 56 | F | Fever, lymphadenopathy | Porta hepatis | No known compromise | Negative | Lymphadenitis | Yes | CD4 T | Polyclonal |
| 8 | 34 | F | Generalized lymphadenopathy | Right axillary | No known compromise | Not done | Lymphadenitis | yes | CD4 T | Polyclonal |
Cases previously published7
M – male, F – female, CHL – classical Hodgkin lymphoma, AITL – angioimmunoblastic T cell lymphoma, TRG – T cell receptor gamma gene rearrangement
Immunohistochemistry was done on 10% neutral buffered formalin fixed paraffin embedded sections using standard immunoperoxidase procedure with an automated immunostainer (Roche Diagnostics Corporation, Indianapolis, IN) per the manufacturer’s instructions. The antibody panel for the cases of reactive lymphadenitis included CD3, CD20, CD25, CD4, CD8, CD79a, PAX-5, Epstein-Barr virus (EBV-LMP1), CD30, CD5, BCL-6 and CD10. The antibodies used in cases of AITLs were, CD3, CD20, CD79a, PAX-5, CD4, CD8, CD2, CD5, CD7, CD30, CD15, PD-1, BCL-6, CD10, MUM1, CD21, CD23, CD15 and CD138. The antibodies used in the case of CHL included CD20, CD3, CD30, CD15, CD45, PAX-5, CD79a, CD57, CD4, CD8, CD5 and EBV-LMP1.
EBER-ISH and immunohistochemical stains for other viruses (CMV, HHV-8, HSV, and EBV) were also performed in reactive lymphadenitis cases. EBER-ISH was performed using EBER1 DNP probe (Roche Diagnostics Corporation, Indianapolis, IN) on an automated stainer (Roche Diagnostics Corporation, Indianapolis, IN) by previously published methods (18).
PCR for T cell receptor gamma (TRG) gene rearrangement on all cases and immunoglobulin (IG) gene rearrangement when the initial diagnosis warranted, were performed to rule out/confirm a lymphoproliferative disorder. DNA was extracted from paraffin embedded tissue sections and PCR amplified for detection of immunoglobulin (IGH and IGk loci) and TRG gene rearrangements as previously described (19) (20) (21). For both reactions, the joining region primer was covalently linked to a fluorescent dye FAM to allow for fluorescence detection. The products were analyzed by capillary electrophoresis on an ABI 3130xl Genetic Analyzer, and electropherograms were analyzed using GeneMapper software version 4.0 (ABI).
Results
Details of the demographics, clinic-pathological findings and molecular findings of the 8 cases with HHV-6 positivity are given in Table 1.
Viral lymphadenitis
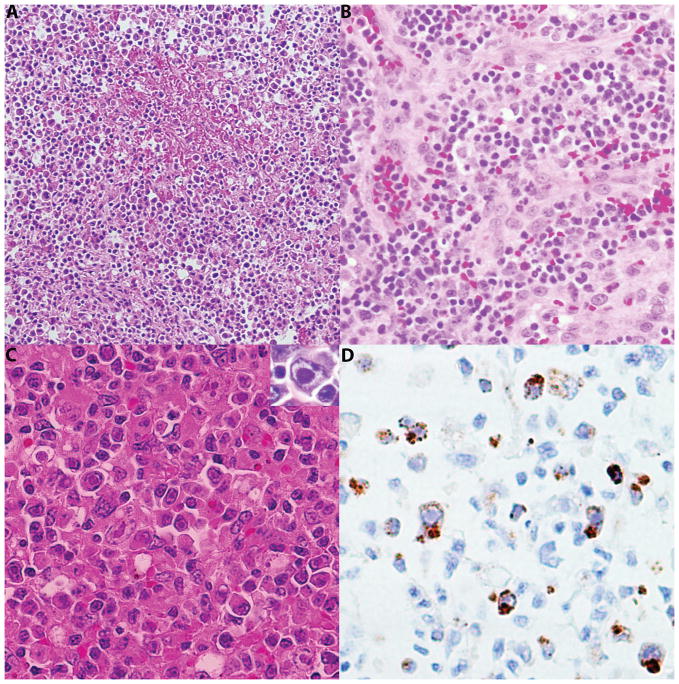
Five cases of reactive lymphadenitis positive for HHV-6 were identified. All patients were adults (median age 53 years; range 28 – 64 years), including 3 males and 2 females. Clinically they presented with generalized lymphadenopathy and fever. None of these patients was known to be immunocompromised. The lymph node excisional biopsies (5/5) showed complete or partial effacement of architecture with marked paracortical hyperplasia. In 4/5 cases diffusely distributed in the paracortex, numerous large atypical lymphoid cells, exhibiting pleomorphic nuclei, vesicular chromatin, and prominent eosinophilic intranuclear inclusions were seen. In one case they formed small clusters. Nevertheless, none of the cases showed isolated single cell with viral inclusions. Vascular proliferation was seen in 4/5 cases and necrosis in 2/5, raising suspicion of peripheral T-cell lymphoma. There were neutrophilic infiltrates including focal microabscesses in all 5 cases. All biopsies were negative for EBV by in situ hybridization. Immunohistochemical stains and/or molecular analysis for other viruses (CMV, HHV-8, HSV and HTLV-1) were negative. The immunohistochemical stains confirmed the morphologic finding of paracortical expansion with a predominance of CD4 positive cells over CD8 positive cells. There were scattered immunoblasts which were highlighted by CD30. The large atypical cells with intranuclear inclusions identified by morphology were positive for HHV6 antibody in a membranous and Golgi pattern, and were also positive for CD3 and CD4. Histologic features of viral lymphadenitis and HHV6 immunohistochemical stain are shown in figure 1.
Figure 1.
Histologic features of viral lymphadenitis. A, Section of the lymph node with fibrinoid necrosis H&E ×100 B, There was significant vascular proliferation H&E ×200 C, Higher power view of the atypical cells with intranuclear eosinophilic inclusions H&E, ×400 C insert, intranuclear inclusion D, Immunohistochemical stain for HHV6 highlighting virally infected cells with membranous and Golgi pattern ×400.
Lymphoproliferative disorders
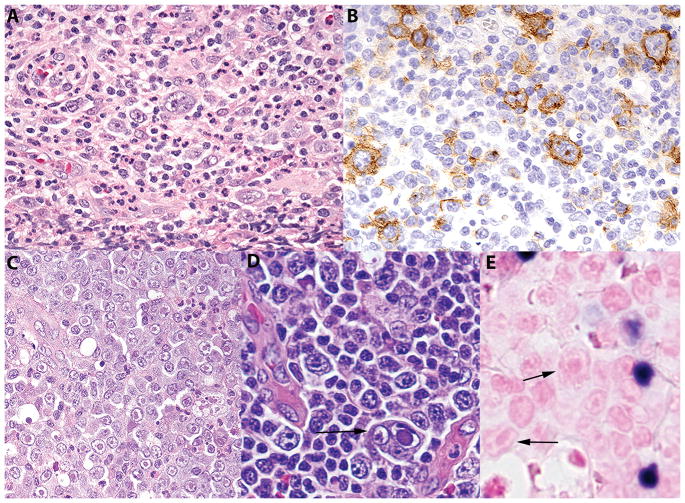
There were two cases of AITL and one case of CHL, nodular sclerosis subtype, which showed non-tumoral lymphocytes positive for HHV-6 by immunohistochemical staining. The patients with AITL were 54 and 60 years of age, both were male and presented with a classical clinical picture of AITL including generalized lymphadenopathy and B symptoms. The patient with CHL was a 17-year-old male, who presented with generalized lymphadenopathy and B symptoms and was found to have lung nodules. These cases showed characteristic morphologic and immunohistochemical findings diagnostic of AITL and CHL (Figure 2A and 2B). Large atypical cells, focally clustered and singly scattered, containing intranuclear and intracytoplasmic inclusions (Figures 2C and 2D) were identified as well. These were associated with increased neutrophils. EBV positive cells were identified in cases of AITL and in the case of CHL the Hodgkin/Reed-Stenberg (HRS) cells were positive for EBV. In AITL the HHV-6 positive cells were T cells as shown by the CD4 (figure 3B) and non tumoral, while EBV positive cells were B cells variable in cell size and often small (Figure 2E). In the CHL case as shown in figure 2C, the virally infected cells tended to be in isolated clusters, which were EBV negative.
Figure 2.
Morphological and immunohistochemical features of CHL and AITL with HHV-6 infection. A, CHL, nodular sclerosis type with mononuclear Hodgkin cells and polymorphous background H&E ×400; B, CHL, the Hodgkin cells are highlighted by CD30 stain ×400; C, CHL, There were focal aggregates of cells with intranuclear eosinophilic viral inclusions similar in morphology to the viral lymphadenitis H&E, ×400; D, AITL, there were large atypical cells with intranuclear viral inclusions similar in morphology to the viral lymphadenitis. Arrow points a multinucleate cell with intranuclear inclusion H&E, ×400; E, AITL, there are small mononuclear EBV positive cells, which are clearly distinct from the virally infected cells (arrows), EBER-ISH, ×400. CHL – classic Hodgkin lymphoma, AITL – Angioimmunoblastic T cell lymphoma, EBER-ISH – Epstein Barr Virus early antigen – in situ hybridization.
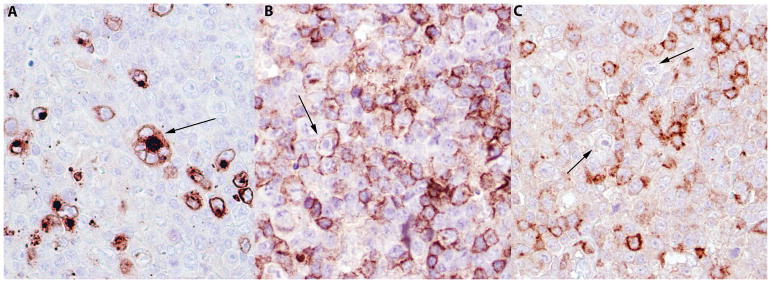
Figure 3.
AITL. A, Immunohistochemical stain for HHV-6 highlighting numerous virally infected cells with a membranous and Golgi pattern. A HHV-6 positive multinucleated cell positive is seen (arrow). B, AITL, Cells with inclusions are also positive for CD4 staining (arrow). C, AITL, the atypical T cells are highlighted by PD-1 positive staining ×400. The cells with viral inclusions were negative for PD-1(arrows) AITL – Angioimmunoblastic T cell lymphoma.
Staining for HHV-6 was positive with a membranous and Golgi pattern (Figure 3A) and was restricted to cells with evident inclusions on H&E. CD3 and CD4 were positive in the cells with viral inclusions (Figure 3B). These cells were immunophenotypically distinguishable from the EBV positive cells in AITL (negative for PD1 (Figure 3C) and CD10) and CHL, and HRS cells (negative for CD30 and CD15) in the case of CHL.
All 20 AITL cases lacking viral inclusions were negative for HHV-6 by immunohistochemistry.
Molecular analysis
PCR for HHV-6 performed on 2 previously reported cases identified HHV-6B species (7).
PCR showed a polyclonal pattern of TRG gene rearrangement in all 3 cases of reactive lymphadenitis with adequate DNA samples. The two cases of AITL showed clonal TRG rearrangement and polyclonal IG gene rearrangement. Molecular analysis was not performed in the case of CHL.
Discussion
HHV-6 can be associated with viral lymphadenitis, and the histological features, if not appreciated, can lead to misdiagnosis. The morphological findings of complete/partial effacement of architecture with marked paracortical expansion, atypical morphology of the lymphoid cells, necrosis and vascular proliferation in HHV-6 infection may raise concern for a diagnosis of lymphoproliferative disorder, particularly peripheral T cell lymphoma. The presence of large atypical cells resembling immunoblasts with prominent eosinophilic intranuclear inclusions may result in a mistaken diagnosis of classical Hodgkin lymphoma. In cases of lymphoma with associated HHV-6 infection, presence of neutrophilic infiltrates and necrosis may obscure the morphology of underlying lymphoma, thus leading to misinterpretation.
Epidemiologic studies using serologic and molecular methods have shown that most people get infected by HHV-6 early in life. Children get primary infection which can be clinically asymptomatic or can be in the form of exanthem subitum or roseola infantum and thus the HHV-6 and HHV-7 viruses are called Roseola viruses. By contrast, primary infection is not common in adults and they are prone to develop reactivation of latent virus particularly in an immunocompromised state (13, 15, 16), although rare cases of primary infection in adults have been reported (6).
In vitro cell culture studies have shown that both HHV-6A and HHV-6B replicate in peripheral blood mononuclear cells and cord blood lymphocytes, particularly T cells (22). Ex vivo studies have shown that even though HHV-6 A and B replicate in human lymphoid tissues, the effect on cellular viability and immunophenotype are different for the two species. Productive infection in both CD4+ and CD8+ T cells is identified; however, HHV-6A is more efficient in infecting CD8+ T cells compared to HHV-6B, while both species depleted CD4+ T cells (23). A loss of surface CD3 expression in HHV-6 infected cells also was identified (22, 23). CD3 plays a major role in antigen recognition and the T cell immune response. Thus, it is possible that HHV-6 can cause dysregulation of T cell immune function. In addition, in vitro studies have shown than HHV-6 infection causes cytopathic effects including ballooning and multinucleated giant cells (24). These findings are in keeping with our findings, as we observed multinucleation and/or binucleation of virally infected cells in reactive lymphadenitis and non tumoral cells in AITL (see Figure 3A). HHV-6 viral inclusions were limited to CD4+ T cells. Moreover, the infected cells showed morphologic atypia in the form of cellular and nuclear enlargement and cytoplasmic and nuclear inclusions, as well as increased apoptosis. Data previously published by other groups have shown that follicular dendritic cells (FDCs) show positive staining with antibodies specific for HHV6 late antigen p101K (17, 25). The cases we have studied did not show positive staining of FDCs with the HHV6 antibody that we used. Moreover, the distribution pattern of the virally infected cells was limited to the paracortex and did not involve secondary follicles or follicular dendritic cells. The receptor used by HHV-6B to enter cells is detected with CD134, a member of the TNF receptor superfamily expressed by T cells (26). This supports our finding of T cells being infected by the virus.
There have been contradictory proposals about the causal role of HHV-6 in lymphoproliferative disorders and in progression over the years (9, 17), particularly as the virus was first isolated from peripheral blood mononuclear cells of patients with lymphoproliferative disorders (1). An association between AITL and HHV-6 has been published by several groups. One group found a positive association of the presence of viral DNA and temporal progression of AITL cases, suggesting that HHV-6 might be causing AITL, aiding disease progression or both (9). Another specific disease association that has been discussed in literature is with CHL (27). However, these studies identified the virus only by PCR and did not perform immunohistochemical stains to identify viral cellular localization. Prior studies using both PCR and immunohistochemical methods suggested that the virus is absent from the neoplastic cells in both Hodgkin and non-Hodgkin lymphomas. (17)
Our findings support this observation. We identified positive immunostaining for HHV-6 only in cases in which the viral inclusions were evident on H&E sections. HRS cells in the case of CHL also were negative for HHV-6 associated protein. Cells staining for HHV-6 showed similar morphology in all 8 cases - enlarged immunoblast-like cells with large vesicular nuclei and intranuclear +/− intracytoplasmic inclusions.
The antibody we used is against the viral envelope glycoprotein (gp60/110kDa) and thus highlights only the cells in which the virus is replicating and producing viral particles and not cells with latent infection. Thus, this stain might be underestimating the cases with latent HHV-6 infection. The theory that HHV-6 is a silent bystander in lymphoproliferative disorders is supported by the presence of serologic evidence of past infection and molecular detection of HHV-6 DNA in both neoplastic and normal tissues in a majority of the population (15–17). However, it cannot be entirely excluded that the immunosuppressive effect of HHV-6 is causing changes in the tumor microenvironment and thus causing progression of the disease22, 23. Alternatively, viral infection of bystander T-cells might expand secondary to decreased immune surveillance in patients with advanced disease.
Of interest, all three cases of lymphoma also contained EBV positive cells, which were distinguishable from the HHV-6 positive cells by morphology and in-situ hybridization (see Figure 2E). The co-existence of EBV and HHV-6 in AITL has been described before (9). None of the 3/5 viral lymphadenitis cases in our cohort in which EBER-ISH was done, was positive for EBV. EBV and HHV-6, both belong to the herpes virus family but belonging to different subfamilies and have different biology and pathogenetic mechanisms. Nevertheless, co-infection in patients with lymphoma suggests decreased immune surveillance as a possible cause.
In conclusion, HHV-6, particularly HHV-6B can be identified in various lymphoid pathologies including Hodgkin and non-Hodgkin lymphomas as well as reactive lymph nodes. It is important to identify the viral inclusions in infected cells, which should lead to further evaluation using immunohistochemical methods, molecular analysis and possibly serology. Failure to properly identify the inclusions could lead to diagnostic pitfalls including: 1) failure to identify the presence of infection in cases of viral lymphadenitis 2) misinterpretation of viral lymphadenitis as lymphoma, particularly T cell lymphoma or even as Hodgkin lymphoma 3) failure to recognize the presence of infection in cases otherwise characteristic of lymphoma.
Acknowledgments
This study was supported by funding from the intramural program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
We acknowledge the contribution of pathologists who submit cases for consultation and the technical support of Theresa Davies-Hill and the molecular diagnostic laboratory.
Footnotes
Disclosures: The author(s) have no conflicts of interest or funding to disclose
Disclosure
This study was presented in part as a poster at the United States and Canadian Academy of Pathology annual meeting 2018 at Vancouver, Canada.
References
- 1.Salahuddin SZ, Ablashi DV, Markham PD, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science (New York, NY) 1986;234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 2.Yamanishi K, Okuno T, Shiraki K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet (London, England) 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 3.Ablashi DV, Salahuddin SZ, Josephs SF, et al. HBLV (or HHV-6) in human cell lines. Nature. 1987;329:207. doi: 10.1038/329207a0. [DOI] [PubMed] [Google Scholar]
- 4.Irving WL, Cunningham AL. Serological diagnosis of infection with human herpesvirus type 6. BMJ (Clinical research ed) 1990;300:156–159. doi: 10.1136/bmj.300.6718.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merelli E, Sola P, Barozzi P, et al. An encephalitic episode in a multiple sclerosis patient with human herpesvirus 6 latent infection. Journal of the neurological sciences. 1996;137:42–46. doi: 10.1016/0022-510x(95)00319-w. [DOI] [PubMed] [Google Scholar]
- 6.Akashi K, Eizuru Y, Sumiyoshi Y, et al. Brief report: severe infectious mononucleosis-like syndrome and primary human herpesvirus 6 infection in an adult. The New England journal of medicine. 1993;329:168–171. doi: 10.1056/NEJM199307153290304. [DOI] [PubMed] [Google Scholar]
- 7.Maric I, Bryant R, Abu-Asab M, et al. Human herpesvirus-6-associated acute lymphadenitis in immunocompetent adults. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2004;17:1427–1433. doi: 10.1038/modpathol.3800179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y, Wang Z, Sun K, et al. HHV-6-associated acute lymphadenitis in immunocompetent patients: a case report and review of literature. International journal of clinical and experimental pathology. 2014;7:3413–3417. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Attygalle AD, Chuang SS, et al. Angioimmunoblastic T-cell lymphoma: histological progression associates with EBV and HHV6B viral load. British journal of haematology. 2007;138:44–53. doi: 10.1111/j.1365-2141.2007.06620.x. [DOI] [PubMed] [Google Scholar]
- 10.Ablashi D, Agut H, Alvarez-Lafuente R, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Archives of virology. 2014;159:863–870. doi: 10.1007/s00705-013-1902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isegawa Y, Mukai T, Nakano K, et al. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. Journal of virology. 1999;73:8053–8063. doi: 10.1128/jvi.73.10.8053-8063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewhurst S, McIntyre K, Schnabel K, et al. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. infants. Journal of clinical microbiology. 1993;31:416–418. doi: 10.1128/jcm.31.2.416-418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. The New England journal of medicine. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 14.Bates M, Monze M, Bima H, et al. Predominant human herpesvirus 6 variant A infant infections in an HIV-1 endemic region of Sub-Saharan Africa. Journal of medical virology. 2009;81:779–789. doi: 10.1002/jmv.21455. [DOI] [PubMed] [Google Scholar]
- 15.Cuende JI, Ruiz J, Civeira MP, et al. High prevalence of HHV-6 DNA in peripheral blood mononuclear cells of healthy individuals detected by nested-PCR. Journal of medical virology. 1994;43:115–118. doi: 10.1002/jmv.1890430203. [DOI] [PubMed] [Google Scholar]
- 16.Okuno T, Takahashi K, Balachandra K, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. Journal of clinical microbiology. 1989;27:651–653. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luppi M, Barozzi P, Garber R, et al. Expression of human herpesvirus-6 antigens in benign and malignant lymphoproliferative diseases. The American journal of pathology. 1998;153:815–823. doi: 10.1016/S0002-9440(10)65623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawnicki LC, Rubocki RJ, Chan WC, et al. The distribution of gene segments in T-cell receptor gamma gene rearrangements demonstrates the need for multiple primer sets. The Journal of molecular diagnostics : JMD. 2003;5:82–87. doi: 10.1016/s1525-1578(10)60456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. Journal of clinical pathology. 1992;45:770–775. doi: 10.1136/jcp.45.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 22.Lusso P, Malnati M, De Maria A, et al. Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. Journal of immunology (Baltimore, Md : 1950) 1991;147:685–691. [PubMed] [Google Scholar]
- 23.Grivel JC, Santoro F, Chen S, et al. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. Journal of virology. 2003;77:8280–8289. doi: 10.1128/JVI.77.15.8280-8289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori Y, Yamanishi K. HHV-6A, 6B, and 7: pathogenesis, host response, and clinical disease. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. Copyright (c) Cambridge University Press 2007. [PubMed] [Google Scholar]
- 25.Forghieri F, Luppi M, Barozzi P, et al. Chronic and recurrent benign lymphadenopathy without constitutional symptoms associated with human herpesvirus-6B reactivation. British journal of haematology. 2016;172:561–572. doi: 10.1111/bjh.13871. [DOI] [PubMed] [Google Scholar]
- 26.Tang H, Serada S, Kawabata A, et al. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9096–9099. doi: 10.1073/pnas.1305187110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torelli G, Marasca R, Luppi M, et al. Human herpesvirus-6 in human lymphomas: identification of specific sequences in Hodgkin’s lymphomas by polymerase chain reaction. Blood. 1991;77:2251–2258. [PubMed] [Google Scholar]