Fig. 1.
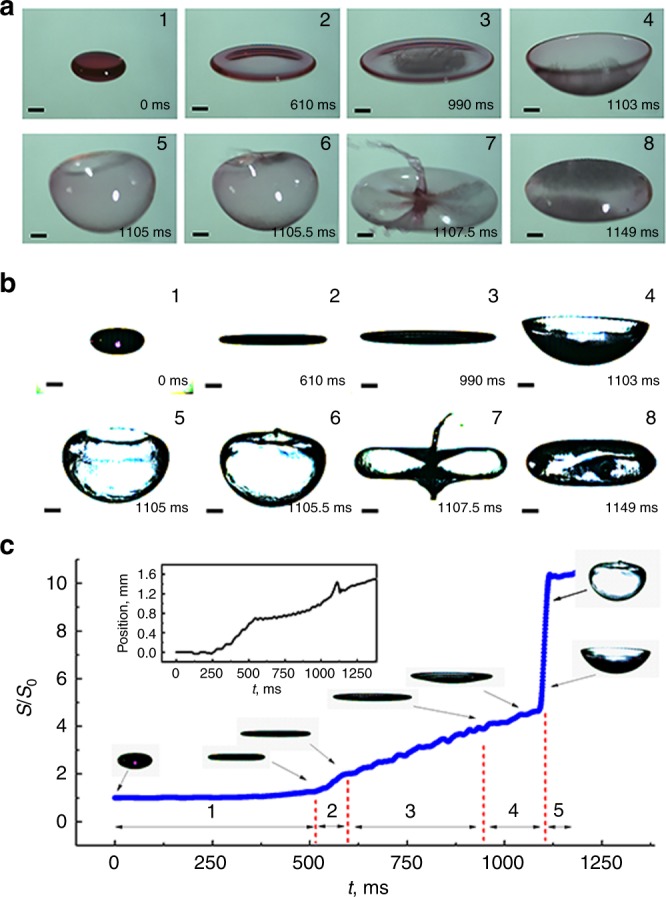
Drop-to-bubble transition of acoustically levitated drop. The process was triggered via increasing sound intensity through decreasing the emitter–reflector distance at a rate uR = 1.50 mm/s. a Snapshots (taken with a high-speed camera titled at an angle of ~35°) of the evolution of a levitated oblate drop (0 ms) of aqueous sodium dodecyl sulfate (SDS) solution at its critical micelle concentration, CMC (~2.3 g/L). Upon increasing the sound intensity, the drop flattens (610 ms) and buckles (990 ms). The buckled liquid film then expands and its rim retracts inward (1103–1105.5 ms), forming a closed bubble (1149 ms). Liquid jetting is shown in 1107.5 ms. To enhance visibility, the drop was dyed with a commercial red ink. The volume of the drop is 10 μL. Each scale bar represents 1 mm. b Side-view snapshots of the same process as shown in a. c Surface area (S) variation of the drop with time divided into five stages: (1) slight deformation, (2) rapid flattening, (3) slow flattening, (4) buckling, and (5) abrupt expansion with rim closure. Inset photos show side-view snapshots corresponding to each stage. Inset graphics shows the levitation position (the initial drop centroid was defined as zero) of the drop/bubble was uplifted slightly (~1.5 mm) because of the lift of the nodal plane caused by the decrease of the emitter–reflector distance. The surface area is scaled to the initial surface area (S0) of a spherical drop
