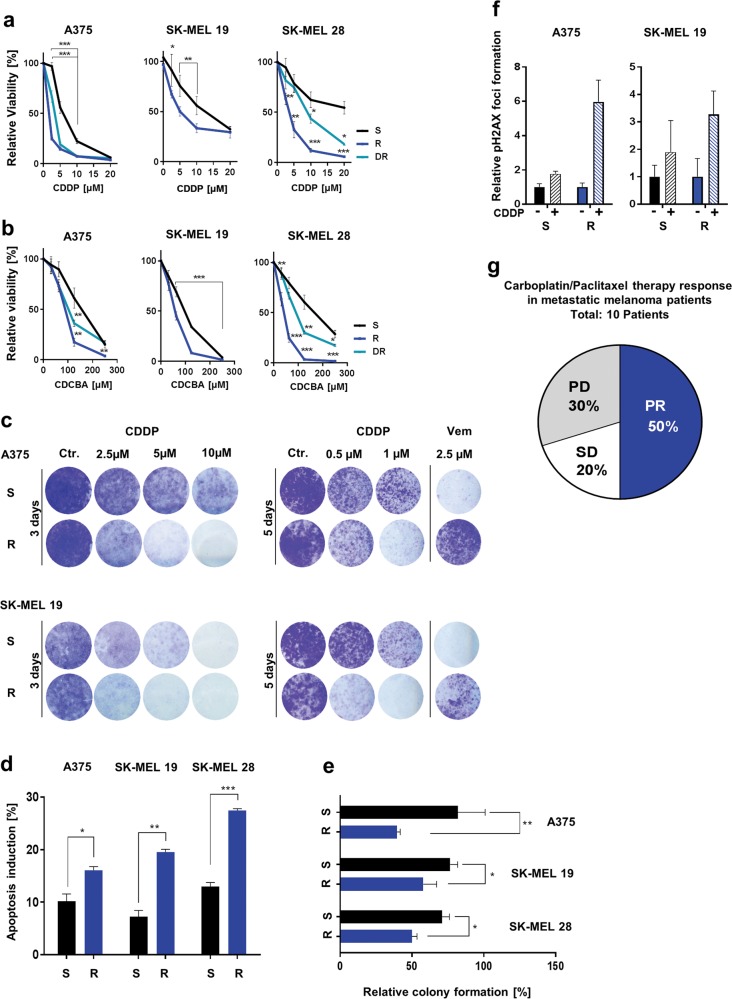
Fig. 1. MAPKi-resistant melanoma cells are susceptible to cisplatin and carboplatin treatment.
a, b Viability analysis after the treatment of treatment-naive (S), BRAFi-resistant (R) and BRAFi and MEKi double-resistant (DR) melanoma cells with cisplatin (CDDP, up to 20 µM) (a) or carboplatin (CDCBA, up to 250 µM) for 72 h (b). The viability signals were normalized to untreated cell signals. (Mean values ± SD, n = 3) c Clonogenic assay of S and R melanoma cells after cisplatin (CDDP) treatment for 3 or 5 days (crystal violet staining). d Percentage of apoptosis induction 3 days after cisplatin (5 µM) treatment of S and R melanoma cells. (Mean values ± SD, n = 3). e Relative number of cell colonies formed in soft agar 12 days after cisplatin treatment (5 µM) by S and R melanoma cells. The colony numbers were normalized to the number of colonies formed by corresponding untreated cells. (Mean values ± SD, n = 3). f pH2AX foci number per cell counted in samples of S and R melanoma cells after cisplatin treatment (CDDP, 10 µM) for 24 h. pH2AX foci number per cell was normalized to the number of foci per untreated control cell (Ctr.). (Mean values ± SD; n = 3). g Patient response to carboplatin/paclitaxel therapy denoted as progressive disease (PD), stable disease (SD) or partial response (PR) was analysed using data of ten melanoma patients. All patients received a previous targeted therapy shown in Suppl. Figure 2a