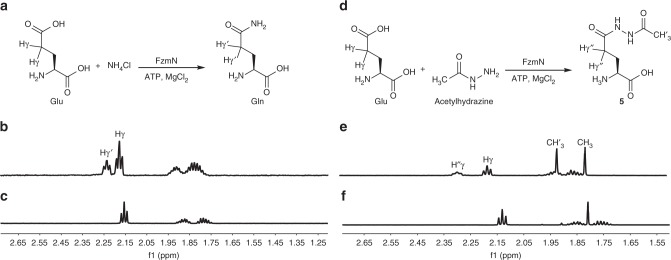
Fig. 4.
1H NMR analysis of FzmN activity. a The canonical glutamine synthetase reaction catalyzed by FzmN. The γ-protons on the Glu substrate (Hγ) and Gln product (Hγ’) are highlighted. b 1H NMR analysis of a reaction mixture containing FzmN, Glu, NH4Cl, ATP, and MgCl2. Peaks corresponding to the γ-protons on Glu and Gln are indicated. c 1H NMR analysis of the reaction mixture containing Glu, NH4Cl, ATP, and MgCl2 without FzmN. d The putative reaction catalyzed by FzmN during the biosynthesis of fosfazinomycin. The γ-protons on the Glu substrate (γ) and the glutamylacetylhydrazine (5) product (γ”) and the methyl protons on the acetylhydrazine substrate (CH3) and 5 (CH’3) are indicated. e 1H NMR analysis of the reaction mixture containing FzmN, Glu, acetylhydrazine, ATP, and MgCl2. Resonances corresponding to the γ-protons and methyl protons on the substrates and the product are indicated. f 1H NMR analysis of the reaction mixture containing Glu, acetylhydrazine, ATP, and MgCl2 without FzmN. The chemical shifts of several of the protons are sensitive to conditions (pH, salt), and hence spiking with authentic materials was used for assignments