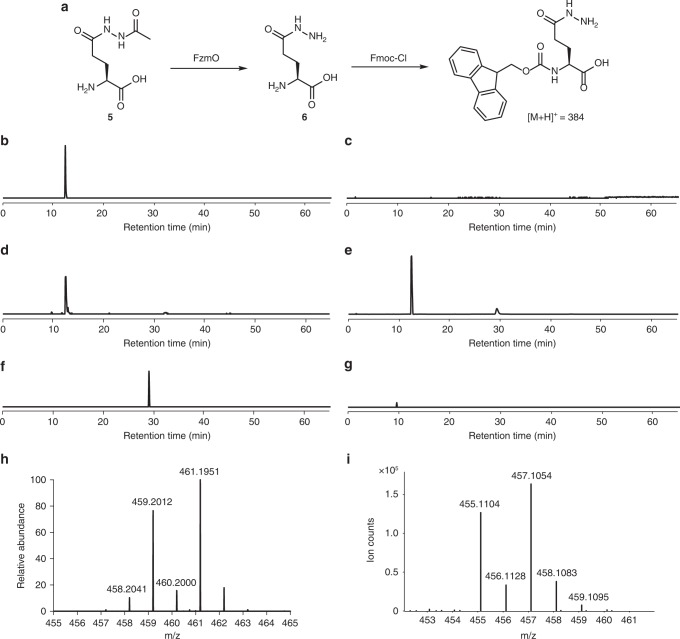
Fig. 5.
FzmO converts glutamylacetylhydrazine (5) to glutamylhydrazine (6). All samples were derivatized with Fmoc-Cl, and the mass of Fmoc-derivatized 6 ([M+H]+ = 384) was monitored by LC-MS. a Deacetylation of 5 to 6 by FzmO in vitro. b Extracted ion chromatogram (EIC) for m/z 384 of the reaction containing FzmO and 5. c EIC for m/z = 384 of a sample containing 5 without FzmO. d EIC for m/z 384 of a synthetic standard of Fmoc-6. e EIC for m/z 384 of a synthetic standard of Fmoc-6 spiked into the reaction mixture containing FzmO and 5 in panel b. f EIC for m/z 368 of Fmoc-derivatized Glu standard. g EIC for m/z 368 of the reaction mixture of FzmO and glutamylacetylhydrazine. h HRMS analysis of 14N incorporation into 15N-labeled fosfazinomycin A in Streptomyces sp. NRRL S-149 fed with unlabeled 6. The calculated m/z ([M+H]+) for 15N-fosfazinomycin A containing two 14N atoms is 459.2025. i HRMS analysis of 15N incorporation into kinamycin D from feeding 0.2 mM of 15N2-6 to cultures of S. murayamaensis. 15N2-kinamycin D ([M+H]+ = 457.1026)