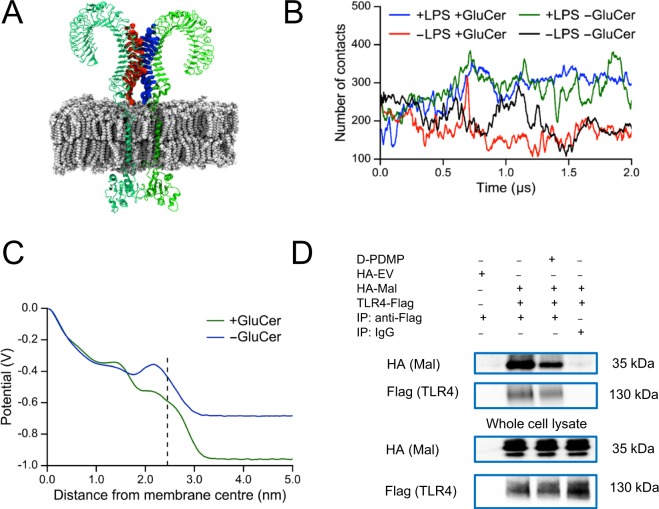
Figure 4.
GluCer does not affect dimer integrity but alters electrostatic potential and TLR4 interaction with the intracellular signaling molecule Mal. (A) Snapshot of the TLR4 complex showing the regions in the extracellular domain used to calculate the number of contacts between the monomers. A contact was defined as a pair of atoms, each one on a different monomer, within a distance of 0.6 nm. (B) Number of contacts between the extracellular domain of the two monomers of TLR4 throughout the whole simulation (2 µs) for 1 run in the presence and absence of (1) LPS in the MD-2 binding pocket and (2) GluCer in the membrane. Mean ± EE values over the last 1 µs of simulation for 3 runs: 337 ± 16 (+LPS +GluCer); 165 ± 14 (−LPS +GluCer); 322 ± 16 (+LPS −GluCer); 187 ± 50 (−LPS −GluCer; 1 run). (C) Profile of the electrostatic potential calculated for a symmetric membrane (with the same lipid composition as the outer leaflet of the membrane used in our molecular dynamics simulations of the TLR4 complex) in the presence and absence of GluCer. The dotted line indicates the start of the water phase. The centre of mass of the upright TLR4 extracellular domain is at about 6.5 nm from the membrane centre. (D) Representative immunoblots (from two separate experiments) showing co-immunopreciptation of TLR4 with the adaptor Mal. HEK-Blue cells stably transfected with CD14 and MD-2 were treated with 10 µM D-PDMP or ethanol as vehicle for 3 h. Thereafter, the cells were transfected with 8 µg empty vector plasmid HA-EV, or plasmids encoding HA-Mal (4 µg) and TLR4-Flag (4 µg) for 24 h in the continued presence of D-PDMP or vehicle. Thereafter, cells were treated with 100 ng/ml LPS E. coli O111:B4 for 10 min. The images are the crops of the expanded blots presented in Supplementary Fig. S11. IP, immunoprecipitation.