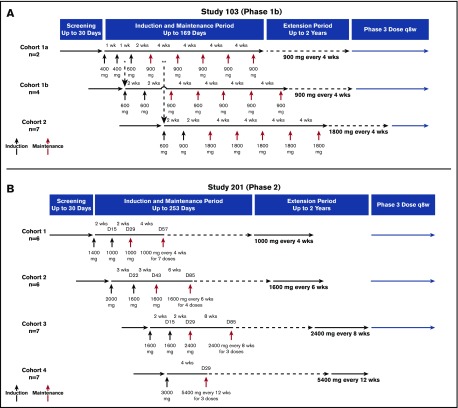
Figure 1.
Overview of study designs. (A) Study 103. Patients in cohort 1a (n = 2) received ravulizumab 400 mg on days 1 and 8, 600 mg on day 15, and 900 mg administered every 28 days beginning on day 29 for a total of 5 maintenance doses. Patients in cohort 1b (n = 4) received ravulizumab 600 mg on days 1 and 15, and then 900 mg administered every 28 days beginning on day 29 for a total of 5 maintenance doses. Patients in cohort 2 (n = 7) received ravulizumab 600 mg on day 1, 900 mg on day 15, and then 1800 mg administered every 28 days beginning on day 29 for a total of 5 maintenance doses. After receiving 5 maintenance doses, each cohort entered a 2-year extension period at the same dosage and frequency as during the maintenance period. *Enrollment into cohort 1b began after the first two 400-mg doses were confirmed safe and tolerable in the first 2 patients. **Enrollment into cohort 2 began 14 days after the first maintenance dose of 900 mg was administered to the second patient in cohort 1a and a data monitoring committee reviewed cumulative safety and tolerability data and confirmed no identified safety risks. (B) Study 201. Patients in cohort 1 (n = 6) received ravulizumab1400 mg on day 1, 1000 mg on day 15, and then 1000 mg administered every 28 days beginning on day 29 for a total of 8 maintenance doses. Patients in cohort 2 (n = 6) received ravulizumab 2000 mg on day 1, 1600 mg on day 22, and then 1600 mg every 42 days beginning on day 43 for a total of 5 maintenance doses. Patients in cohort 3 (n = 7) received ravulizumab 1600 mg on days 1 and 15, and then 2400 mg every 56 days beginning on day 29 for a total of 4 maintenance doses. Patients in cohort 4 (n = 7) received ravulizumab 3000 mg on day 1, and then 5400 mg every 84 days for a total of 3 maintenance doses. After the treatment period, each cohort entered a 2-year extension period at the same dosage and frequency that was received during the treatment period. D, day; q8w, every 8 weeks; wks, weeks.