Figure 3.
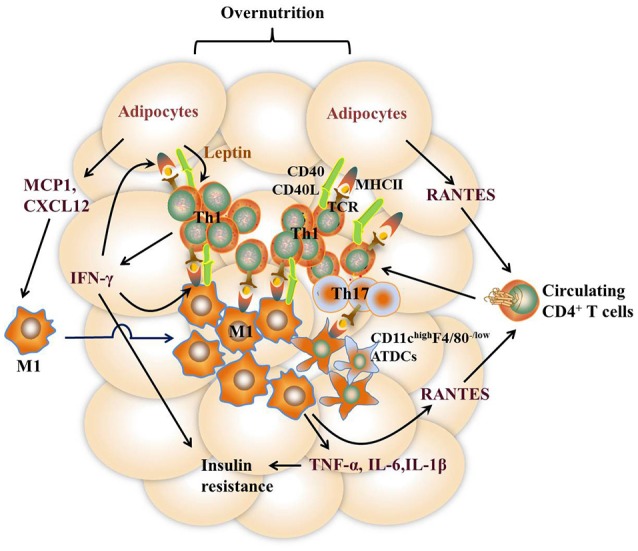
Th1 and Th17 cell-mediated immune responses in VAT under the obese state. Overnutrition causes adipocyte hypertrophy, leading to the release of chemokines such as RANTES, which recruit proinflammatory CD4+ T cell accumulation in VAT via its receptor CCR5. Leptin secreted by adipocytes stimulates IFN-γ production from CD4+ T cells, which further promotes adipocyte MHCII expression and antigen-presentation to induce Th1 cell differentiation, leading to a vicious cycle of AT inflammation. Dead and neighboring adipocytes recruit M1 macrophages to WAT by producing inflammatory mediators such as MCP1 and CXCL12. Likewise, the expression of MHCII in M1 macrophages is promoted by IFN-γ, thus facilitating M1 macrophage-mediated antigen-presentation to induce Th1 cell differentiation. In addition, diet-induced obesity also promotes the expression of inflammatory receptor CD40 expression on ATMs and adipocytes as well as CD40L on CD4+ T cells, which reinforce the crosstalk between CD4 + T cells and these APCs. CD11c highF4/80 low ATDCs are also regarded as APCs to induce Th17 reactivation via production of TGF-β, IL-6, and IL-23. Type 1 cytokines such as TNF-α and IFN-γ, act directly on adipocytes to impair insulin action. Together with IL-6 and IL-1β, these cytokines elicit sustained chronic inflammation that eventually leads to insulin resistance.
