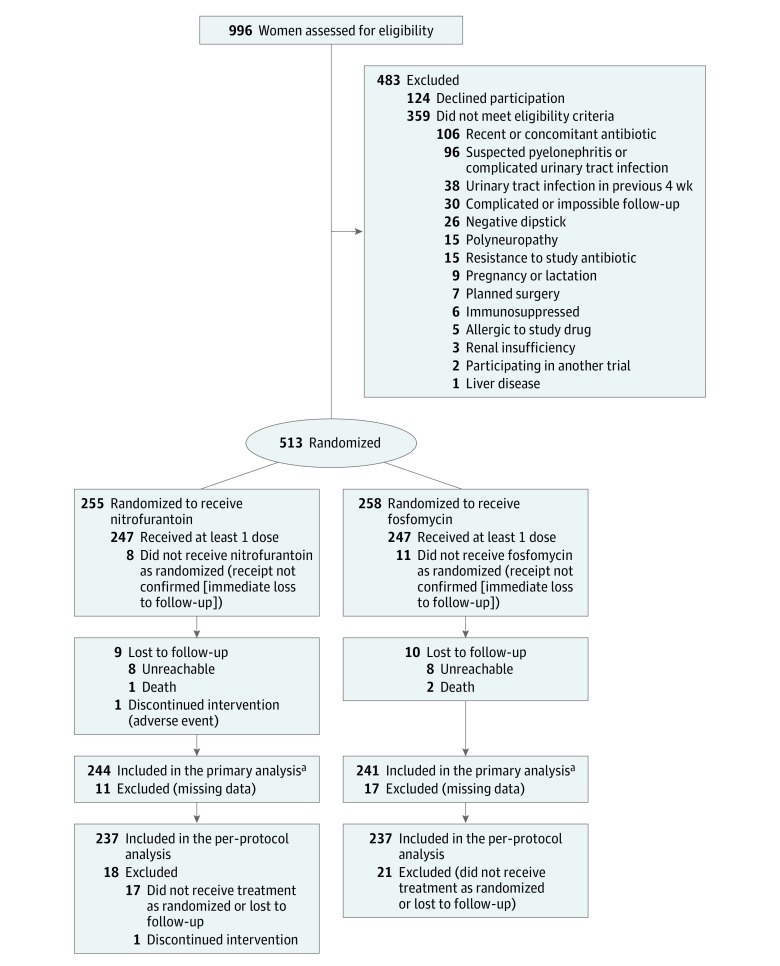
Figure. Study Flowchart of the Nitrofurantoin and Fosfomycin Groups.
Both the intention-to-treat and per-protocol populations were analyzed. The intention-to-treat population included all patients randomized and the per-protocol population, those with at least 80% medication adherence, no major protocol deviations, and available primary outcome data.
aData on the primary outcome were available for 244 of 255 patients (96%) randomized to nitrofurantoin and 241 of 258 patients (93%) randomized to fosfomycin; 7 and 4 patients in these groups, respectively, had clinical failure before being lost to follow-up. The only major protocol deviation documented in either group was nonadherence to the study antibiotic.