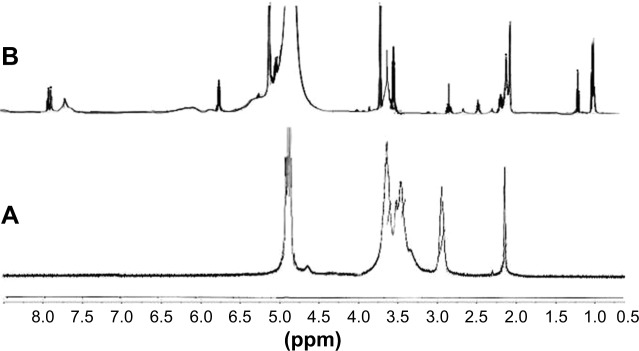
Figure 3.
1H NMR spectra of both Cs and CPP-PEG-Cs confirming successful covalent conjugation with CPP; (A) native Cs, (B) peptide-grafted derivative of Cs (CPP-PEG-Cs).
Notes: The chemical shift at δ 6.7–8.2 belongs to the aromatic protons of the phenyl alanine moiety, which is present in the structure of CPP conjugated to Cs (B); multiple peaks of oxymethyl groups in PEG at δ 3.3–3.7 cover the signals of protons related to pyranose ring of Cs in spectra (A). The characteristic peak at δ 2.05 is related to protons of methoxy groups of Cs as seen in both spectra. The multiple peaks at δ 1.1–1.4 in spectra (B) are from the −CH2−CH2−CH2−NH−NH−NH2 in arginine amino acid in the CPP.
Abbreviations: CPP, cell-penetrating peptide; Cs, chitosan; 1H NMR, 1H nuclear magnetic resonance; PzEG, polyethylene glycol.