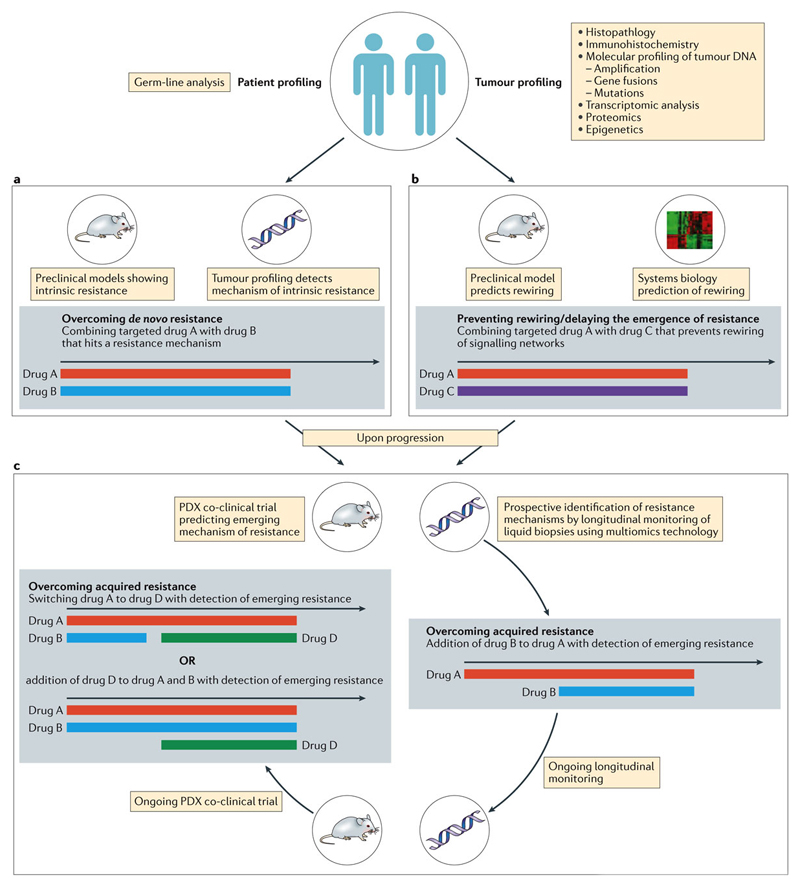
Figure 3. The future: proposed clinical trial designs to overcome resistance.
Patients are their tumours are profiled using -omics technology as shown and various strategies proposed for each hypothetical scenario A) Should profiling indicate a degree of de-novo resistance to the proposed Drug A, in which case, could it be reversed by addition of Drug B, and the combination used up-front. B) Should profiling indicate sensitivity to Drug A, but preclinical models and system biology predict the eventual rewiring of signalling networks causing resistance, Drug A can be combined with Drug C that has a broader epigenetic role. C) Co-clinical strategies employing patient-derived xenografts alongside prospective longitudinal monitoring of circulating cell-free DNA (cfDNA) in patients treated with Drug A. At the earliest sign of the development of resistant clones, Drug B is added in combination with continuation of the PDXs in a co-clinical trial and cfDNA monitoring. With emergence of an alternative mechanism of acquired resistance, Drug D can be added to the cocktail of Drug A & B, or the patient could be switched to a combination of Drug A & D. The black arrows are timelines, with the coloured bars indicating the various drugs described as colour coded.
[Au: I have removed Box 1 as this is just repeating the subheadings in the text. However, I think a Box of glossary items could be useful. Suggested terms include synthetic lethality, Synergistic Additive etc.