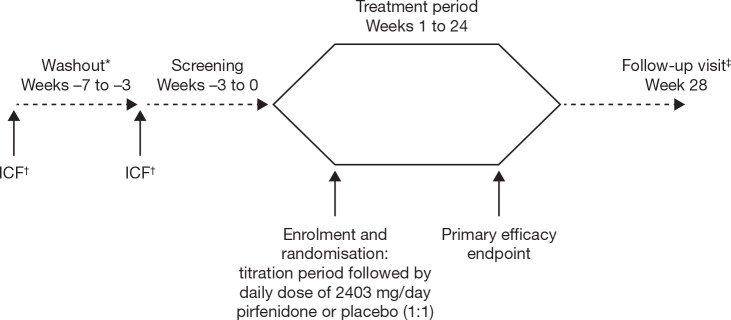
Figure 1.
Study design. *Washout period for study participants taking prohibited medications prior to screening; patients not taking a prohibited medication will forgo the washout period and directly enter screening. †Informed consent may be obtained either at the washout (if applicable) or screening visits and must be obtained before any trial-specific screening procedure is performed. ‡After completion of the treatment period and follow-up visit, study participants will be given the opportunity to take part in an open-label extension of pirfenidone for up to 12 months; a final follow-up visit will be performed 4 weeks after the last open-label dose of pirfenidone. ICF, informed consent form.