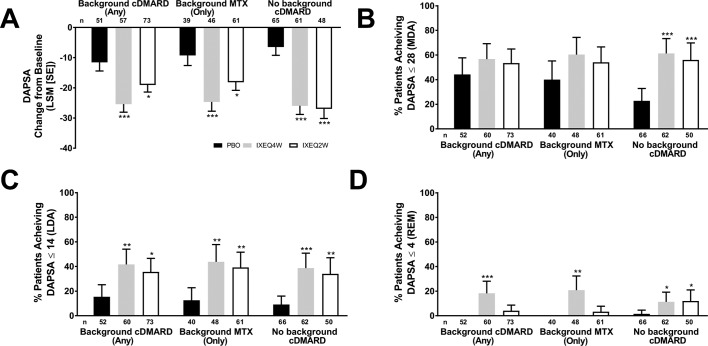
Figure 2.
DAPSA LSM change from baseline (A), patients achieving DAPSA ≤28 (MDA) (B), DAPSA ≤14 (LDA) (C) or DAPSA ≤4 (REM) (D) after 24 weeks in patients treated with PBO, IXEQ4W or IXEQ2W alone or when added to background cDMARDs or MTX. DAPSA ≤28 (MDA) and DAPSA ≤14 (LDA) were calculated in a cumulative manner, thus if a patient was classified as achieving DAPSA ≤28 (MDA), the patient may also have achieved DAPSA ≤14 (LDA) and DAPSA ≤4 (REM). Percentages reported with 95% confidence intervals. The treatment-by-cDMARD use interaction p-value for DAPSA was 0.064, DAPSA ≤28 was 0.060, DAPSA ≤14 was 0.730, and DAPSA ≤4 was 0.049. *p<0.05, **p<0.01, ***p<0.001, all versus PBO. cDMARD, conventional disease-modifying antirheumatic drugs; DAPSA, Disease Activity Index for Psoriatic Arthritis; DAPSA ≤28 (MDA), DASPA moderate disease activity; DAPSA ≤14 (LDA), DAPSA low disease activity; DAPSA ≤4 (REM), DAPSA remission; IXEQ4W, ixekizumab every 4 weeks; IXEQ2W, ixekizumab every 2 weeks; LSM, least squares mean; MTX, methotrexate; n, number of patients; PBO, placebo.