Abstract
Asymmetric aldol addition of simple aldehydes and ketones to electrophiles is a cornerstone reaction for the synthesis of unusual sugars and chiral building blocks. We investigated d-fructose-6-phosphate aldolase from E. coli (FSA) D6X variants as catalysts for the aldol additions of ethanal and nonfunctionalized linear and cyclic aliphatic ketones as nucleophiles to nonphosphorylated hydroxyaldehydes. Thus, addition of propanone, cyclobutanone, cyclopentanone, or ethanal to 3-hydroxypropanal or (S)- or (R)-3-hydroxybutanal catalyzed by FSA D6H and D6Q variants furnished rare deoxysugars in 8–77% isolated yields with high stereoselectivity (97:3 dr and >95% ee).
Keywords: biocatalysis, aldol reaction, aldolases, asymmetric catalysis, deoxysugars, carbon−carbon bond formation, enzyme engineering
Introduction
Crossed aldol additions of simple aliphatic aldehydes and ketones to electrophiles are important transformations in organic synthesis. The asymmetric version enables access to a range of interesting unusual carbohydrates and important chiral building blocks for the preparation of naturally occurring and synthetic bioactive products, including pharmaceuticals.1−8 These conversions are, however, cumbersome because of notorious side reactions such as polymerization, homo aldolization, and aldol condensation, as well as low stereoselectivity.6,9,10 Enzymatic C–C bond formation mediated by aldolases is a very attractive alternative for this purpose, because of its unparalleled high stereoselectivity, avoidance of extensive protective group chemistry, and operation under mild conditions.11−14 A typical disadvantage of these enzymes is their rather narrow scope of acceptable nucleophilic substrates, whereas they tolerate a broad variety of aldehydes as the electrophilic components.
The class I d-fructose-6-phosphate aldolase from E. coli (FSA, EC 4.1.2.−) and variants with minimal mutations on selected residues within its active site, showed an unprecedented tolerance toward a large structural variety of nucleophilic and electrophilic substrates.15−30 This unveiled its extraordinary malleability beyond the boundaries of currently known aldolases. Furthermore, we recently uncovered that ethanal, propanone, butanone, and cyclopentanone are acceptable as nucleophilic components for wild-type FSA and its D6H variant when l- and d-glyceraldehyde-3-phosphate (G3P) are the electrophiles.31 Nevertheless, the corresponding nonphosphorylated analogues reacted sluggishly or failed to furnish an aldol adduct.
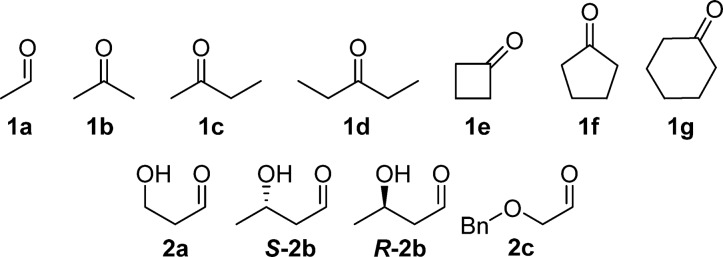
In this work, we show that engineered variants FSA D6X32 catalyze aldol additions of ethanal (1a), linear (1b–d), and cyclic (1e–g) simple aliphatic ketones to nonphosphorylated hydroxyaldehyde derivatives (2a–c) (Figure 1), expanding the synthetic utility to rare and unusual sugar derivatives as well as related chiral building blocks.
Figure 1.
Panel of selected nucleophiles (1a–g) and electrophiles (2a–c).
Results and Discussion
Models of FSA wild-type in complex with the K85-d-fructose-6-phosphate (Fru6P) imine and the K85-dihydroxyacetone (DHA) enamine plus d-G3P show that these substrate intermediates can establish direct contacts with Y131, N28, and D6 residues of the active site (Figure 2). These models suggested that residue D6 can interact via hydrogen-bonding both with the CH2OH group of nucleophiles such as DHA and with the C2-OH of d-G3P, thereby influencing both the nucleophile and electrophile selectivity.33−35 To break the hydrogen bonding pattern of D6, this residue was targeted for site directed mutagenesis to obtain FSA variants with tolerance toward aldol additions of 1a and ketones 1b–g as nucleophiles to nonphosphorylated hydroxyaldehydes. Thus, individual FSA D6X variants were produced, where X = hydrophobic (A and L), polar charged (E and H), or polar uncharged (N, Q, S, and T) amino acids. Variants L107A, L163A, and A165G, which had been identified in earlier studies to benefit the nucleophile and electrophile substrate tolerance, were also screened.16,22
Figure 2.

Molecular models of FSA-bound DHA-enamine/d-G3P (A) and Fru6P-imine (B) intermediates. In both cases, the D6 residue is forming two hydrogen bonds with the C2 hydroxyl group of the d-G3P and the hydroxymethyl group of the DHA (A), or the corresponding C3 and C5 hydroxyl groups of Fru6P (B).
In the standard aldol addition of DHA to d,l-G3P, only the activity of FSA D6N was found to be comparable to that of FSA wild-type (45.4 ± 0.1 U mg–1 vs 46.0 ± 0.1 U mg–1, respectively). Indeed, asparagine is isosteric to aspartate and able to establish similar hydrogen bonding interactions, which explains why no dramatic changes on the activity were observed. On the other hand, for all other D6X replacements, only very low activity was detected (between 0.72 ± 0.01 to 0.03 ± 0.01 U mg–1) (Table S2 in SI), confirming the critical importance of aspartate for binding of hydroxylated nucleophiles.
We began testing the panel of ketone nucleophiles 1b–g, in a concentration range between 100 mM to 20% v/v, optimized for each nucleophile, with a selection of 3-hydroxyaldehyde electrophiles 2a,b (80 mM) (Scheme 1) using FSA D6X variants as catalysts. Electrophiles 2a,b have the advantage to shift reaction equilibrium by cyclic hemiketal formation of the product. Moreover, 2b would introduce a chiral center as internal stereochemical reference (Scheme 1). We started with 3-hydroxypropanal (2a) and the ketone nucleophiles in aqueous 50 mM triethanolamine buffer pH 8, similar to the conditions used in our previous communication.31 D6X variants where X = A, T, or H were identified as the most effective ones for the addition of 1b (2.0 M, 15% v/v) to 2a, based on TLC analysis (Table S3). Compound 3b was isolated in 32% yield using FSA D6H as catalyst (Scheme 1A, Table 1). No reaction was detected for the addition of 1c–g to 2a with any of the FSA variants screened. Increasing the concentration of 1c–g up to 20% v/v, to compensate for potentially low KM values, was unfruitful. Furthermore, in a second round of protein engineering we combined L107A, L163A, and A165G mutations with selected D6X mutations, i.e. X = L, H, or E. However, no positive hits were found (Table S3).
Scheme 1. FSA Variants Catalyzed Aldol Additions of 1a-b, 1e-f to (A) 2a, (B) S-2b, (C) R-2b, and (D) 2c.
Table 1. FSA-Variant-Catalyzed Aldol Additions of 1a,b,e,f, to 2a–c.
| Nu | E | FSA variant | product | isolated yielda % | ee or drb, % |
|---|---|---|---|---|---|
| 1a | 2a | D6L/A165G | 3ac | 4 | –e |
| 1b | 2a | D6H | 3bd | 32 | >95f |
| 1a | S-2b | D6H | 4ag | 27 | 97:3 |
| 1b | S-2b | D6H | 4b | 25 | 97:3 |
| 1e | S-2b | D6H | 4eh | 22 | 97:3 |
| 1f | S-2b | D6H | 4f | 8 | 97:3 |
| 1a | R-2b | D6H | 5ai | 14 | 97:3 |
| 1b | R-2b | D6H | 5b | 13 | 97:3 |
| 1b | 2c | D6Q | 6b | 77 | >98j |
| 1e | 2c | D6Q | 6e:7e | 10 | 75:25 |
Product isolation and purification procedures were devised to eliminate the unconverted starting material and collecting all potential diasteroisomers. Reaction and purification conditions were not optimized.
dr determined by NMR.
α:β 1:1.
α and β anomers in equilibrium with the acyclic structure: α:β:acyclic 0.5:0.9:1.0.
ee not determined, [α]D20= −2.93 (c = 0.62 in MeOH), and this was compared with the optical rotation of the product obtained using DERAEcoli as catalyst: [α]D20= −28.6 (c = 1 in MeOH) (Lit.:3 [α]D20= −19.0 (c = 0.5, MeOH) obtained using also DERAEcoli as catalyst).
ee determined by chiral GC after formation of the methyl glycoside derivative against a racemic sample obtained by aldol addition of 1b (80% v/v) to 2a catalyzed by pyrrolidine.
α:β 1:1.
Hemiketal:acyclic adduct ratio was 85:15.
α:β 4:1.
ee determined by chiral HPLC analysis. Racemic mixture obtained by chemical synthesis using LDA methodology. Nu: nucleophile; E: electrophile.
(S)-3-Hydroxybutanal (S-2b) gave intense new spots on TLC in the aldol additions of: 1b (15% v/v) using FSA D6X, where X = A, L, N, Q, S, T, E, and H; 1e (5% v/v) using FSA D6L and D6H; and 1f (5% v/v) using FSA D6N and D6H (Table S3). The common active variant, FSA D6H, furnished 4b, 4e, and 4f in 25%, 22%, and 8% isolated yields, respectively (Scheme 1B, Table 1).
(R)-3-Hydroxybutanal (R-2b) was tested with 1b (15% v/v) to check whether it was also substrate of FSA D6X catalysts. The best variant (i.e., FSA D6H) gave the expected lactol 5b (Scheme 1C, Table 1). However, lower yields were achieved as compared with S-2b, suggesting some degree of enantiomeric discrimination.31
Next, we studied the addition of ethanal (1a) to electrophiles 2a,b. It was observed that test reactions carried out at 15%–20% v/v of 1a yielded a mixture of products arising from its nonenzymatic self-aldolization.36 It is likely that the electrophilic reactivity of 1a inactivated the enzyme and just the spontaneous chemical reaction took place. Indeed, at 100 mM concentration of 1a, the addition to S- and R-2b catalyzed by FSA D6H and D6N yielded the desired aldol product. The common active variant, FSA D6H, furnished 4a and 5a in 27% and 14% isolated yields, respectively (Scheme 1B,C, Table 1). In the case of 2a, the double mutant FSA D6L/A165G was active for the addition of 1a at 5% v/v, rendering 3a in 4% isolated yield (Table 1).
2-Hydroxyethanal was also tested as electrophile with all nucleophiles 1a-g, but it was not converted in the desired sense by any FSA variant. Thus, the O-protected derivative, 2-benzyloxyethanal (2c), was considered as synthetic equivalent, which we reported as a good electrophile for aldol additions of DHA and HA catalyzed by FSA.22,25 For additions to 2c, D6N and D6Q variants gave the highest conversions (95%) when the electrophile was 1b (15% v/v), whereas only FSA D6Q gave acceptable conversions (53%) when it was 1e (5% v/v) (Table S3). Accordingly, FSA D6Q furnished 6b in 77% and 6e+7e in 10% isolated yields (Scheme 1D, Table 1). No product was formed for all other nucleophiles with any of the FSA variants.
Butanone (1c), 3-pentanone (1d) , and cyclohexanone (1g) were not accepted as nucleophiles by any of the FSA variants assayed with any of the electrophiles. Most probably this is due to steric interactions that difficult a proper orientation for the electrophiles and/or the nucleophiles. It is noteworthy that 1c was tolerated as nucleophile with l-G3P electrophile;31 however the latter is one of the most reactive electrophiles, which could compensate for the lower activity of 1c.
In none of the reactions involving 1a as nucleophile, neither homoaldol addition nor trimerization reactions were observed. This is in contrast with what was seen with 2-deoxy-d-ribose-5-phosphate aldolase Escherichia coli (DERAEcoli).37 Furthermore, no reactions were detected with any nucleophile/electrophile pair in control experiments performed with FSA wild-type and the inactive the inactive FSA K85M variant.
Stereoselectivity is an issue of paramount importance in these reactions. Formation of d-threo aldol adducts has been reported using catalysis by wild-type FSA and variants, practically irrespective of structural variations of nucleophile and electrophile. Moreover, the attack of FSA-enamine nucleophile invariably takes place from its si-face, for steric reasons and with no exceptions hitherto known.18,19,21,24,28,29
Aldol adducts 4a–f and 5a,b, obtained by FSA D6H catalysis showed high diastereomeric purity (dr: 97:3, within the limits of high field NMR detection) (Table 1). The internal chiral center from electrophiles 2b and the formation of 6-membered ring hemiketals helped us to assign the absolute stereochemistry of the one or two newly formed stereocenters, which resulted to be R-configured, in 4a,b and 5a,b, and R,R in 4e–f. Derivatives of lactol 5a are extensively used as the chiral side chain for the synthesis of statin drugs.37 The spectral data of 5a matched those obtained from the trimerization of acetaldehyde catalyzed by DERAEcoli. Lactol 4a is the 5-epimer of 5a and may constitute also an important building block for stereochemical analogs of statins.38 Structural motives depicted in Scheme 1 are present in many natural products and synthetic intermediates.8 Furthermore, rare sugars are important for generating novel “glycol-randomized” analogues of natural products,5,39−41 in the search for improved pharmaceutical lead compounds.42
Consistent with the stereochemical outcome of adducts 4e–f, an E configured FSA K85-enamine is imposed by the rigid ring of nucleophiles 1e and 1f (Figure 3).31 In contrast, a Z configured FSA K85-enamine nucleophile is always formed with the acyclic hydroxymethylketone analogues (Figure 1A).17−19,28 In both cases, the nucleophilic attack takes place from the si-face, and the C=O of both S- and R-2b was always attacked from its si-face. Thus, the anti-configured aldol adducts were obtained with nucleophiles 1e and 1f, whereas the syn products were observed with acyclic hydroxymethylketones. The anti-stereochemistry from the addition of cyclopentanone to l-G3P catalyzed by FSA D6H, could not be unequivocally ascertained in the previous work because of the epimerization of the C-α to the carbonyl stereocenter under the reaction conditions.31 In the present case, the epimerization was plausibly precluded by the in situ formation of the cyclic hemiketals: for 4e the cyclic:acyclic ratio was 85:15, and for 4f, no acyclic species were detected by NMR.
Figure 3.

Molecular models of FSA-bound 1e-enamine/S-2b (A) and 1f-enamine/S-2b (B) intermediates.
Products 3b and 6b, synthesized using catalysis by FSA D6H and D6Q, respectively, showed high enantiomeric purity by chiral GC and HPLC analysis against racemic samples (Table 1). By analogy with 4b, it may safely be assumed that 3b has the R configuration, which correlates to products 4a and 5a,b. This is consistent with the addition of hydroxymethylketone nucleophiles to 2a catalyzed by wild-type FSA.17,28 Moreover, the optical rotation of the unprotected product 9b, [α]D20= −30.3 (c = 0.5 in CHCl3) (lit.:43 for the R enantiomer [α]D20 = +34.0 (c = 0.44, CHCl3)) indicated that it was the S enantiomer.
The aldol addition of 1e to 2c gave a mixture of two diastereoisomers 6e:7e. These can arise from (i) the lack of a precise stereofacial C=O orientation of 2c to the enzyme-enamine complex, yielding epimers at C-β to the carbonyl,19,26 or (ii) the epimerization of the C-α to the carbonyl group (i.e., the carbon tertiary center and in this case no hemiketal can be formed) under the reaction conditions (pH 8.0).31 Since deuterium exchange at C-α to the carbonyl group was not detected by NMR, it was concluded that products 6e and 7e were obtained via the first route.
Conclusions
We herein present FSA D6X variants that catalyze aldol additions of ethanal (1a) and simple ketones 1b, 1e, and 1f to nonphosphorylated hydroxyaldehydes (2c,d). Substitution of D6 in wild-type FSA by amino acids such as L, N, Q, E, or H is critical to generate favorable interactions at the active site for nucleophile activation. It is noteworthy that different residues in this position with dissimilar electronic and steric properties, but not the native aspartate, rendered enzymes that were able to catalyze the addition. Because the isosteric neutral mutation D6N was active, aspartate appears to have a detrimental electronic effect for the addition of nonfunctionalized aliphatic ketones and ethanal to nonphosphorylated electrophilic substrates. Propanone (1b) was accepted as nucleophile component with all the selected electrophiles. Propanone is the closest analogue of hydroxypropanone, the best reported hydroxymethylketone nucleophile substrate for FSA.24,25,28 The FSA D6H variant was effective with most of the reactions, accepting structurally different nucleophiles (e.g., 1a and 1f). Since none of the nucleophiles or electrophiles studied here has equivalent hydroxyl groups to those present in hydroxyketones, which can establish hydrogen-bond interactions with residue D6 (Figure 2), it could be speculated that H in position 6 could have a role limiting the conformational mobility of substrates because of its larger size. This could pose a hindrance to the rotation of the C–N enamine bond and favor an adequate orientation of the approaching acceptor carbonyl, thus favoring the reaction of selected substrates.
We demonstrate that direct biocatalytic bottom up construction of rare deoxysugars and chiral intermediates is possible by the choice of aldol components using FSA as catalyst with immediate control over sites of deoxygenation. This work, along with previous studies on FSA, has shown the unprecedented versatility of this aldolase with just minimal changes in its active site. In this sense, FSA goes far beyond any known aldolase in its breath of scope for asymmetric organic synthesis, particularly when compared with dihydroxyacetone phosphate (DHAP)-dependent aldolases and DERA for their narrow nucleophile selectivity.44−48 DERA is highly selective for ethanal but showed two-orders of magnitude lower activity toward ketones such as propanone and fluoropropanone.48 Further experiments to widen the synthetic scope of FSA for aldol reactions of simple aliphatic aldehydes and ketones to amino aldehydes are in progress.
Acknowledgments
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 635595 (CarbaZymes), the Ministerio de Economía y Competitividad (MINECO), the Fondo Europeo de Desarrollo Regional (FEDER) (grant CTQ2015-63563-R to P.C. and CTQ2015-64436-P to T.P.), COST action CM1303 Systems Biocatalysis and funding from the Auvergne council.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.8b02486.
Materials, general procedures, protein expression and purification of FSA catalysts and 2-deoxy-d-ribose-5-phosphate aldolase from E. coli (DERAEcoli) catalyst, activity assay of FSA catalysts, preparation of racemic aldol adducts; FSA variants screening; preparative enzymatic reactions, description of physical and spectral properties and NMR spectra of the corresponding aldol adducts (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
European Union’s Horizon 2020 grant agreement no 635595 (CarbaZymes). Ministerio de Economía y Competitividad (MINECO) and Fondo Europeo de Desarrollo Regional (FEDER) (grant CTQ2015–63563-R to P.C. and CTQ2015–64436-P to T.P.). Funding from the Auvergne (France) council.
The authors declare no competing financial interest.
Supplementary Material
References
- Hoffmann R. W. Conformation Design of Open-Chain Compounds. Angew. Chem., Int. Ed. 2000, 39, 2054–2070. . [DOI] [PubMed] [Google Scholar]
- Fleming I.; Ghosh S. K. Stereocontrol in Organic Synthesis using Silicon-Containing Compounds. Syntheses of (±)-2-Deoxyribonolactone and (±)-arabonolactone. J. Chem. Soc., Perkin Trans. 1 1998, 2711–2720. 10.1039/a804282i. [DOI] [Google Scholar]
- Liu J. J.; Wong C. H. Aldolase-Catalyzed Asymmetric Synthesis of Novel Pyranose Synthons as a New Entry to Heterocycles and Epothilones. Angew. Chem., Int. Ed. 2002, 41, 1404–1407. . [DOI] [PubMed] [Google Scholar]
- He X. M.; Liu H.-w. Formation of Unusual Sugars: Mechanistic Studies and Biosynthetic Applications. Annu. Rev. Biochem. 2002, 71, 701–754. 10.1146/annurev.biochem.71.110601.135339. [DOI] [PubMed] [Google Scholar]
- Thibodeaux C. J.; Melançon C. E. I.; Liu H.-w. Natural-Product Sugar Biosynthesis and Enzymatic Glycodiversification. Angew. Chem., Int. Ed. 2008, 47, 9814–9859. 10.1002/anie.200801204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez A.; Zumbansen K.; Döhring A.; van Gemmeren M.; List B. Improved Conditions for the Proline-Catalyzed Aldol Reaction of Acetone with Aliphatic Aldehydes. Synlett 2014, 25, 932–934. 10.1055/s-0033-1340919. [DOI] [Google Scholar]
- Mahrwald R.; Gündogan B. Highly Regioselective Lewis Acid-Mediated Aldol Additions at the More Encumbered α-Side of Unsymmetrical Ketones. J. Am. Chem. Soc. 1998, 120, 413–414. 10.1021/ja973453l. [DOI] [Google Scholar]
- Schetter B.; Mahrwald R. Modern Aldol Methods for the Total Synthesis of Polyketides. Angew. Chem., Int. Ed. 2006, 45, 7506–7525. 10.1002/anie.200602780. [DOI] [PubMed] [Google Scholar]
- Scheffler U.; Mahrwald R. Asymmetric Organocatalyzed Direct Aldol Additions of Enolizable Aldehydes. Synlett 2011, 2011, 1660–1667. 10.1055/s-0030-1260813. [DOI] [Google Scholar]
- Northrup A. B.; MacMillan D. W. C. The First Direct and Enantioselective Cross-Aldol Reaction of Aldehydes. J. Am. Chem. Soc. 2002, 124, 6798–6799. 10.1021/ja0262378. [DOI] [PubMed] [Google Scholar]
- Clapés P.; Fessner W.-D.; Sprenger G. A.; Samland A. K. Recent Progress in Stereoselective Synthesis with Aldolases. Curr. Opin. Chem. Biol. 2010, 14, 154–167. 10.1016/j.cbpa.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Dean S. M.; Greenberg W. A.; Wong C.-H. Recent Advances in Aldolase-Catalyzed Asymmetric Synthesis. Adv. Synth. Catal. 2007, 349, 1308–1320. 10.1002/adsc.200700115. [DOI] [Google Scholar]
- Clapés P.Enzymatic C–C Bond Formation. In Biocatalysis in Organic Synthesis; Faber K., Fessner W.-D., Turner N. J., Eds.; Georg Thieme Verlag KG: Stuttgart (Germany), 2015; Vol. 2, pp 31–92. [Google Scholar]
- Wechsler C.; Meyer D.; Loschonsky S.; Funk L.-M.; Neumann P.; Ficner R.; Brodhun F.; Müller M.; Tittmann K. Tuning and Switching Enantioselectivity of Asymmetric Carboligation in an Enzyme through Mutational Analysis of a Single Hot Spot. ChemBioChem 2015, 16, 2580–2584. 10.1002/cbic.201500529. [DOI] [PubMed] [Google Scholar]
- Schürmann M.; Sprenger G. A. Fructose-6-Phosphate Aldolase is a Novel Class I Aldolase from Escherichia coli and is Related to a Novel Group of Bacterial Transaldolases. J. Biol. Chem. 2001, 276, 11055–11061. 10.1074/jbc.M008061200. [DOI] [PubMed] [Google Scholar]
- Güclü D.; Szekrenyi A.; Garrabou X.; Kickstein M.; Junker S.; Clapés P.; Fessner W.-D. Minimalist Protein Engineering of an Aldolase Provokes Unprecedented Substrate Promiscuity. ACS Catal. 2016, 6, 1848–1852. 10.1021/acscatal.5b02805. [DOI] [Google Scholar]
- Szekrenyi A.; Garrabou X.; Parella T.; Joglar J.; Bujons J.; Clapés P. Asymmetric Assembly of Aldose Carbohydrates from Formaldehyde and Glycolaldehyde by Tandem Biocatalytic Aldol Reactions. Nat. Chem. 2015, 7, 724–729. 10.1038/nchem.2321. [DOI] [PubMed] [Google Scholar]
- Soler A.; Gutiérrez M. L.; Bujons J.; Parella T.; Minguillon C.; Joglar J.; Clapés P. Structure-Guided Engineering of d-Fructose-6-Phosphate Aldolase for Improved Acceptor Tolerance in Biocatalytic Aldol Additions. Adv. Synth. Catal. 2015, 357, 1787–1807. 10.1002/adsc.201500073. [DOI] [Google Scholar]
- Szekrenyi A.; Soler A.; Garrabou X.; Guerard-Helaine C.; Parella T.; Joglar J.; Lemaire M.; Bujons J.; Clapés P. Engineering the Donor Selectivity of d-Fructose-6-Phosphate Aldolase for Biocatalytic Asymmetric Cross-Aldol Additions of Glycolaldehyde. Chem. - Eur. J. 2014, 20, 12572–12583. 10.1002/chem.201403281. [DOI] [PubMed] [Google Scholar]
- Soler A.; Garrabou X.; Hernández K.; Gutiérrez M. L.; Busto E.; Bujons J.; Parella T.; Joglar J.; Clapés P. Sequential Biocatalytic Aldol Reactions in Multistep Asymmetric Synthesis: Pipecolic Acid, Piperidine and Pyrrolidine (Homo)Iminocyclitol Derivatives from Achiral Building Blocks. Adv. Synth. Catal. 2014, 356, 3007–3024. 10.1002/adsc.201400453. [DOI] [Google Scholar]
- Guerard-Helaine C.; Debacker M.; Clapes P.; Szekrenyi A.; Helaine V.; Lemaire M. Efficient Biocatalytic Processes for Highly Valuable Terminally Phosphorylated C5 to C9 D-Ketoses. Green Chem. 2014, 16, 1109–1113. 10.1039/C3GC42140F. [DOI] [Google Scholar]
- Gutierrez M.; Parella T.; Joglar J.; Bujons J.; Clapés P. Structure-Guided Redesign of d-Fructose-6-phosphate Aldolase from E. coli: Remarkable Activity and Selectivity Towards Acceptor Substrates by Two-Point Mutation. Chem. Commun. 2011, 47, 5762–5764. 10.1039/c1cc11069a. [DOI] [PubMed] [Google Scholar]
- Castillo J. A.; Guérard-Hélaine C.; Gutiérrez M.; Garrabou X.; Sancelme M.; Schürmann M.; Inoue T.; Hélaine V.; Charmantray F.; Gefflaut T.; Hecquet L.; Joglar J.; Clapés P.; Sprenger G. A.; Lemaire M. A Mutant d-Fructose-6-Phosphate Aldolase (Ala129Ser) with Improved Affinity towards Dihydroxyacetone for the Synthesis of Polyhydroxylated Compounds. Adv. Synth. Catal. 2010, 352, 1039–1046. 10.1002/adsc.200900772. [DOI] [Google Scholar]
- Garrabou X.; Castillo J. A.; Guérard-Hélaine C.; Parella T.; Joglar J.; Lemaire M.; Clapés P. Asymmetric Self- and Cross-Aldol Reaction of Glycolaldehyde Catalyzed by d-Fructose-6-phosphate Aldolase. Angew. Chem., Int. Ed. 2009, 48, 5521–5525. 10.1002/anie.200902065. [DOI] [PubMed] [Google Scholar]
- Concia A. L.; Lozano C.; Castillo J. A.; Parella T.; Joglar J.; Clapés P. d-Fructose-6-Phosphate Aldolase in Organic Synthesis: Cascade Chemical-Enzymatic Preparation of Sugar-Related Polyhydroxylated Compounds. Chem. - Eur. J. 2009, 15, 3808–3816. 10.1002/chem.200802532. [DOI] [PubMed] [Google Scholar]
- Castillo J. A.; Calveras J.; Casas J.; Mitjans M.; Vinardell M. P.; Parella T.; Inoue T.; Sprenger G. A.; Joglar J.; Clapés P. Fructose-6-Phosphate Aldolase in Organic Synthesis: Preparation of d-Fagomine, N-Alkylated Derivatives, and Preliminary Biological Assays. Org. Lett. 2006, 8, 6067–6070. 10.1021/ol0625482. [DOI] [PubMed] [Google Scholar]
- Fessner W. D.; Heyl D.; Rale M. Multi-Enzymatic Cascade Synthesis of d-Fructose 6-Phosphate and Deoxy analogs as Substrates for High-Throughput Aldolase Screening. Catal. Sci. Technol. 2012, 2, 1596–1601. 10.1039/c2cy20092a. [DOI] [Google Scholar]
- Rale M.; Schneider S.; Sprenger G. A.; Samland A. K.; Fessner W.-D. Broadening Deoxysugar Glycodiversity: Natural and Engineered Transaldolases Unlock a Complementary Substrate Space. Chem. - Eur. J. 2011, 17, 2623–2632. 10.1002/chem.201002942. [DOI] [PubMed] [Google Scholar]
- Hélaine V.; Mahdi R.; Sudhir Babu G. V.; de Berardinis V.; Wohlgemuth R.; Lemaire M.; Guérard-Hélaine C. Straightforward Synthesis of Terminally Phosphorylated l-Sugars via Multienzymatic Cascade Reactions. Adv. Synth. Catal. 2015, 357, 1703–1708. 10.1002/adsc.201500190. [DOI] [Google Scholar]
- Sanchez-Moreno I.; Helaine V.; Poupard N.; Charmantray F.; Legeret B.; Hecquet L.; Garcia-Junceda E.; Wohlgemuth R.; Guerard-Helaine C.; Lemaire M. One-Pot Cascade Reactions using Fructose-6-phosphate Aldolase: Efficient Synthesis of d-Arabinose 5-Phosphate, d-Fructose 6-Phosphate and Analogues. Adv. Synth. Catal. 2012, 354, 1725–1730. 10.1002/adsc.201200150. [DOI] [Google Scholar]
- Roldán R.; Sanchez-Moreno I.; Scheidt T.; Hélaine V.; Lemaire M.; Parella T.; Clapés P.; Fessner W.-D.; Guérard-Hélaine C. Breaking the Dogma of Aldolase Specificity: Simple Aliphatic Ketones and Aldehydes are Nucleophiles for Fructose-6-phosphate Aldolase. Chem. - Eur. J. 2017, 23, 5005–5009. 10.1002/chem.201701020. [DOI] [PubMed] [Google Scholar]
- Junker S.; Roldan R.; Joosten H.-J.; Clapés P.; Fessner W.-D. Complete Switch of Reaction Specificity of an Aldolase by Directed Evolution In Vitro: Synthesis of Generic Aliphatic Aldol Products. Angew. Chem., Int. Ed. 2018, 57, 10153–10157. 10.1002/anie.201804831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehwess-Litzmann A.; Neumann P.; Parthier C.; Lüdtke S.; Golbik R.; Ficner R.; Tittmann K. Twisted Schiff Base Intermediates and Substrate Locale Revise Transaldolase Mechanism. Nat. Chem. Biol. 2011, 7, 678–684. 10.1038/nchembio.633. [DOI] [PubMed] [Google Scholar]
- Stellmacher L.; Sandalova T.; Leptihn S.; Schneider G.; Sprenger G. A.; Samland A. K. Acid–Base Catalyst Discriminates between a Fructose 6-Phosphate Aldolase and a Transaldolase. ChemCatChem 2015, 7, 3140–3151. 10.1002/cctc.201500478. [DOI] [Google Scholar]
- Tittmann K. Sweet Siblings with Different Faces: The Mechanisms of FBP and F6P Aldolase, Transaldolase, Transketolase and Phosphoketolase Revisited in Light of Recent Structural Data. Bioorg. Chem. 2014, 57, 263–280. 10.1016/j.bioorg.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Castañar L.; Roldán R.; Clapés P.; Virgili A.; Parella T. Disentangling Complex Mixtures of Compounds with Near-Identical 1H and 13C NMR Spectra using Pure Shift NMR Spectroscopy. Chem. - Eur. J. 2015, 21, 7682–7685. 10.1002/chem.201500521. [DOI] [PubMed] [Google Scholar]
- Gijsen H. J. M.; Wong C.-H. Unprecedented Asymmetric Aldol Reactions with Three Aldehyde Substrates Catalyzed by 2-Deoxyribose-5-Phosphate Aldolase. J. Am. Chem. Soc. 1994, 116, 8422–8423. 10.1021/ja00097a082. [DOI] [Google Scholar]
- Muller M. Chemoenzymatic Synthesis of Building Blocks for Statin Side Chains. Angew. Chem., Int. Ed. 2005, 44, 362–365. 10.1002/anie.200460852. [DOI] [PubMed] [Google Scholar]
- Thibodeaux C. J.; Melancon C. E.; Liu H.-w. Unusual Sugar Biosynthesis and Natural Product Glycodiversification. Nature (London, U. K.) 2007, 446, 1008–1016. 10.1038/nature05814. [DOI] [PubMed] [Google Scholar]
- Luzhetskyy A.; Mendez C.; Salas J. A.; Bechthold A. Glycosyltransferases, Important Tools for Drug Design. Curr. Top. Med. Chem. 2008, 8, 680–709. 10.2174/156802608784221514. [DOI] [PubMed] [Google Scholar]
- Mendez C.; Luzhetskyy A.; Bechthold A.; Salas J. A. Deoxysugars in Bioactive Natural Products: Development of Novel Derivatives by Altering The Sugar Pattern. Curr. Top. Med. Chem. 2008, 8, 710–724. 10.2174/156802608784221532. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M.; Snader K. M. Natural Products as Sources of New Drugs over the Period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Li D. R.; Murugan A.; Falck J. R. Enantioselective, Organocatalytic Oxy-Michael Addition to γ/δ-Hydroxy-α,β-enones: Boronate-Amine Complexes as Chiral Hydroxide Synthons. J. Am. Chem. Soc. 2008, 130, 46–48. 10.1021/ja076802c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arth H. L.; Fessner W.-D. Practical Synthesis of 4-Hydroxy-3-Oxobutylphosphonic Acid and its Evaluation as a Bio-Isosteric Substrate of DHAP Aldolase. Carbohydr. Res. 1997, 305, 313–321. 10.1016/S0008-6215(97)10026-X. [DOI] [PubMed] [Google Scholar]
- Fessner W.-D.; Sinerius G. Enzymes in Organic Synthesis. 7. Synthesis of Dihydroxyacetone Phosphate (and Isosteric Analogs) by Enzymic Oxidation: Sugars from Glycerol. Angew. Chem., Int. Ed. Engl. 1994, 33, 209–212. 10.1002/anie.199402091. [DOI] [Google Scholar]
- Garrabou X.; Joglar J.; Parella T.; Crehuet R.; Bujons J.; Clapés P. Redesign of the Phosphate Binding Site of l-Rhamnulose-l-Phosphate Aldolase towards a Dihydroxyacetone Dependent Aldolase. Adv. Synth. Catal. 2011, 353, 89–99. 10.1002/adsc.201000719. [DOI] [Google Scholar]
- Garrabou X.; Calveras J.; Joglar J.; Parella T.; Bujons J.; Clapés P. Highly Efficient Aldol additions of DHA and DHAP to N-Cbz-Amino aldehydes Catalyzed by l-Rhamnulose-1-Phosphate and l-Fuculose-1-Phosphate Aldolases in Aqueous Borate Buffer. Org. Biomol. Chem. 2011, 9, 8430–8436. 10.1039/c1ob06263h. [DOI] [PubMed] [Google Scholar]
- Chen L.; Dumas D. P.; Wong C.-H. Deoxyribose 5-Phosphate Aldolase as a Catalyst in Asymmetric Aldol Condensation. J. Am. Chem. Soc. 1992, 114, 741–748. 10.1021/ja00028a050. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.