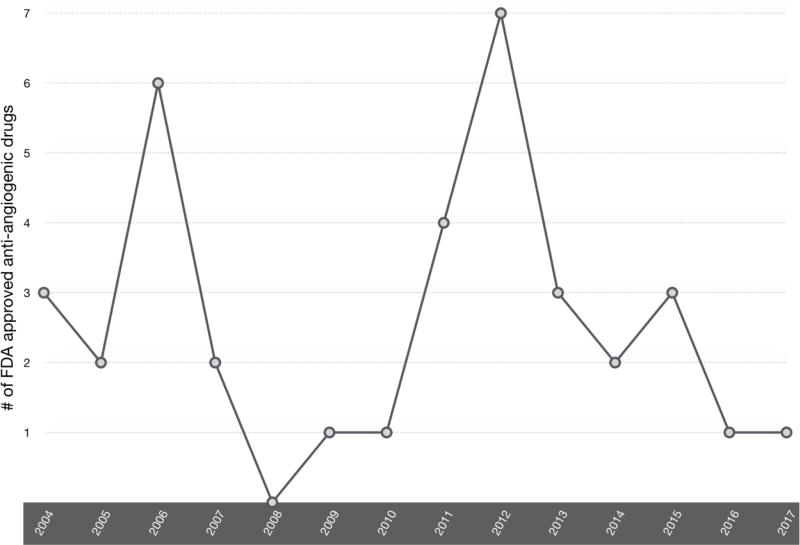
Figure I. Trend in FDA approval of anti-angiogenic therapies.
In 2004, bevacizumab became the first anti-angiogenic therapy approved for the treatment of metastatic colorectal cancer, and the approval of erlotinib and pegaptanib followed shortly after in the same year. Regorafenib, a treatment for hepatocellular carcinoma, is the most recently approved anti-angiogenic therapy. We observe that from 2008 to 2012 there was a steady increase of the number of FDA-approved drugs. A total of 7 anti-angiogenic drugs were approved by the FDA. This steady increase reflects the growing interest in using anti-angiogenic drugs in cancer treatment. There are 26 molecules approved for anti-angiogenic therapy, some with multiple, unique indications. Drugs were counted once per approved indication (e.g., Afatinib is counted for one approval in 2013 and one approval for a separate indication in 2016.)