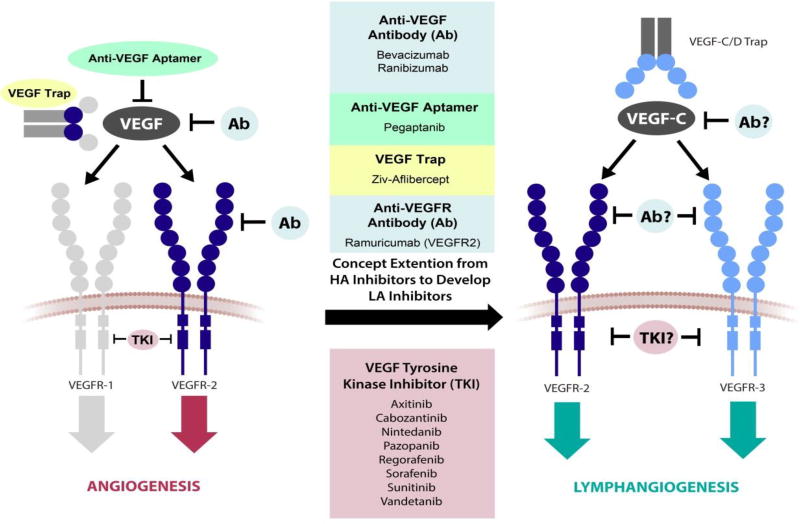
Figure V. Schematic of VEGF pathway target sites of FDA-approved anti-angiogenesis drugs and potential VEGF pathway target sites for future anti-lymphangiogenesis drugs.
Axitinib, Bevacizumab, Cabozantinib, Nintedanib, Pazopanib, Pegaptanib, Ramuricumab, Ranibizumab, Regorafenib, Sorafenib, Sunitinib, and Vandetanib are approved anti-angiogenic therapies that target VEGF-mediated angiogenesis. Inhibition of a VEGF ligand, a VEGFR binding site, or VEGFR tyrosine kinase activity leads to a reduction in angiogenesis. This strategy can potentially be translated for lymphangiogenesis-targeted drug design. Development of anti-lymphangiogenic therapies may follow the design of anti-angiogenic therapies by interrupting the lymphangiogenic factor VEGF-C, its receptors VEGFR2 and VEGFR3, or the tyrosine kinase activity of VEGFR2 and VEGFR3. HA, hemangiogenic; LA, lymphangiogenic.