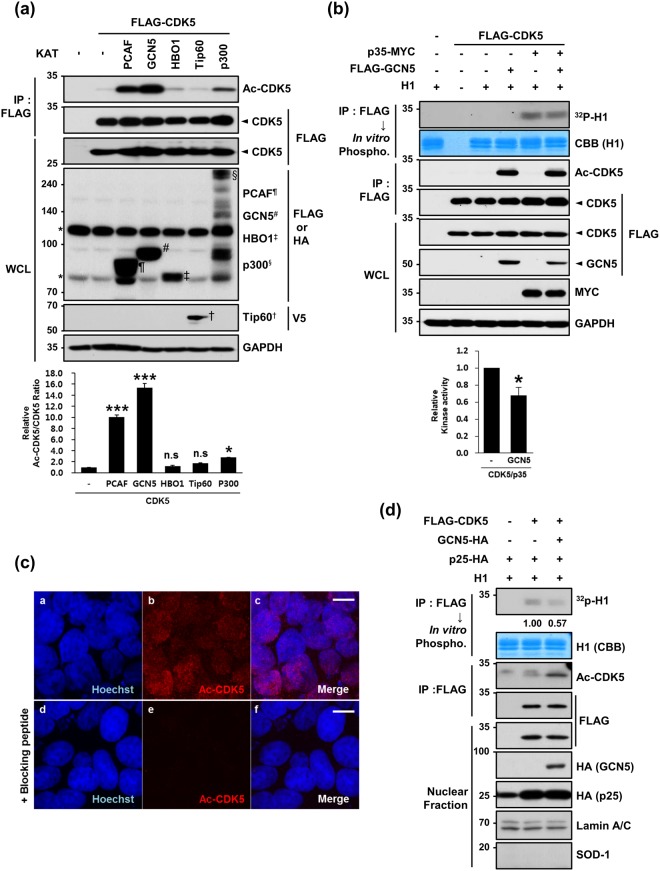
Figure 2.
GCN5 acetylates CDK5 at K33 in the nucleus. (a) Lysates obtained from HEK293 cells expressing FLAG-CDK5 plus one of the indicated KAT vectors were subjected to IP with an anti-FLAG antibody followed by IB with an anti-Ac-CDK5 antibody or anti-FLAG antibody. The intensity of the Ac-CDK5 band was measured using Image-J software and normalized to FLAG-CDK5. The fold change over the control (value = 1) is indicated at the bottom of the blot. WCLs were subjected to IB analysis with the indicated antibodies. Each KAT band is marked by the indicated letters. The asterisk indicates non-specific bands. After normalization to FLAG-CDK5, the fold intensity of Ac-CDK5 versus the control (value = 1) was indicated. The bar represents the mean ± S.D from three independent experiments. ***p < 0.001; *p < 0.05; n.s, not significant. (b) Lysates harvested from HEK293 cells expressing FLAG-CDK5 alone or in combination with p35-MYC and/or FLAG-GCN5 were subjected to IP with an anti-FLAG antibody. The bound CDK5 was incubated in the presence of H1 and [γ-32P]ATP and visualized by autoradiography. The relative kinase activity of CDK5/p35 was expressed as the fold change over the control (value = 1). The bar represents the mean ± S.D from 3 independent experiments. *p < 0.05. (c) HEK293 cells were immunostained with an anti-Ac-CDK5 antibody. Staining specificity was confirmed by pre-incubating with the blocking peptide (EIVAL(acK)RVRLD) that was used to raise the antibody. The nuclei were counterstained with Hoechst dye. Confocal microscopy images are shown. The scale bar represents 10 μm. (d) HEK293 cells transfected with the indicated combinations of constructs were subjected to cellular fractionation. The resulting nuclear fractions were IPed with an anti-FLAG antibody and subsequently subjected to either IB with an anti-Ac-CDK5 or anti-FLAG antibody or an in vitro phosphorylation assay in the presence of H1 and [γ-32P]ATP. Signals from the phosphorylated H1 were visualized by autoradiography. The fold change over the control (value = 1) is indicated. Nuclear fractions were subjected to IB with the indicated antibodies. Anti-SOD-1 and anti-lamin A/C antibodies were employed to verify the purity of the nuclear fractions.