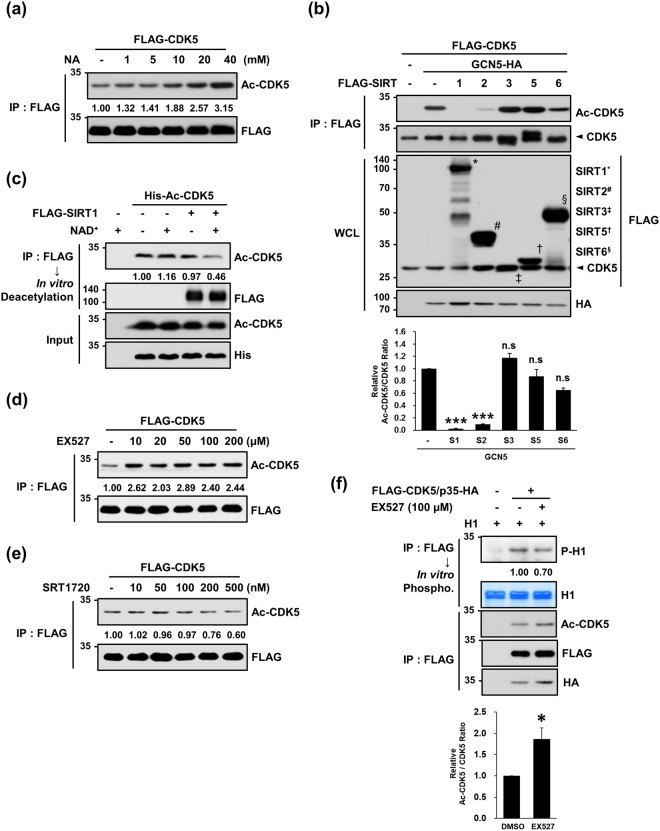
Figure 3.
SIRT1 is responsible for the deacetylation of Ac-CDK5. (a) HEK293 cells transiently expressing FLAG-CDK5 were treated for 24 hrs with increasing doses of nicotinamide (NA, a pan-SIRT inhibitor). Lysates were subjected to IP with an anti-FLAG antibody and probed with the indicated antibodies. After normalization to FLAG-CDK5, the fold intensity of Ac-CDK5 versus control (value = 1) was determined. (b) HEK293 cells were transfected with one of the FLAG-tagged SIRTs plus FLAG-CDK5 and GCN5-HA. Lysates were subjected to IP with an anti-FLAG antibody and IB with an anti-Ac-CDK5 antibody. WCLs were subjected to IB with the indicated antibodies. Each band of SIRTs is marked by the indicated letters. After normalization to FLAG-CDK5, the fold intensity of Ac-CDK5 versus the control (value = 1) was indicated. The bar represents the mean ± S.D from three independent experiments. ***p < 0.001; n.s, not significant. (c) FLAG-SIRT1 was expressed in HEK293 cells and purified by IP with FLAG beads. SIRT1-bound beads were incubated with recombinant His-Ac-CDK5 supplemented with β-nicotinamide adenine dinucleotide (NAD+) to activate SIRT1. Reaction mixtures were subjected to IB with an anti-Ac-CDK5 antibody. The fold intensity of Ac-CDK5 versus the control (value = 1) was determined after normalization to the His-Ac-CDK5 inputs. The inputs were probed with the indicated antibodies. (d,e) HEK293 cells transfected with FLAG-CDK5 were treated with increasing doses of (d) EX527 (a selective SIRT1 inhibitor) or (e) SRT1720 (a selective SIRT1 activator) for 24 hrs. Lysates were subjected to IP with an anti-FLAG antibody followed by IB with an anti-Ac-CDK5 antibody. After normalization to FLAG-CDK5, the fold change over the control (value = 1) was determined. (f) Lysates were prepared from HEK293 cells transfected with FLAG-CDK5 and p35-HA and exposed to 100 μM EX527 for 24 hrs. Immunoprecipitates purified with an anti-FLAG antibody were subjected either to IB with the indicated antibodies or an in vitro phosphorylation assay in the presence of H1 and cold ATP. Phospho-H1 signals were visualized with an anti-phospho-H1 antibody. After normalization to FLAG-CDK5, the fold intensity of Ac-CDK5 versus the control (value = 1) was indicated. The bar represents the mean ± S.D from three independent experiments. *p < 0.05.