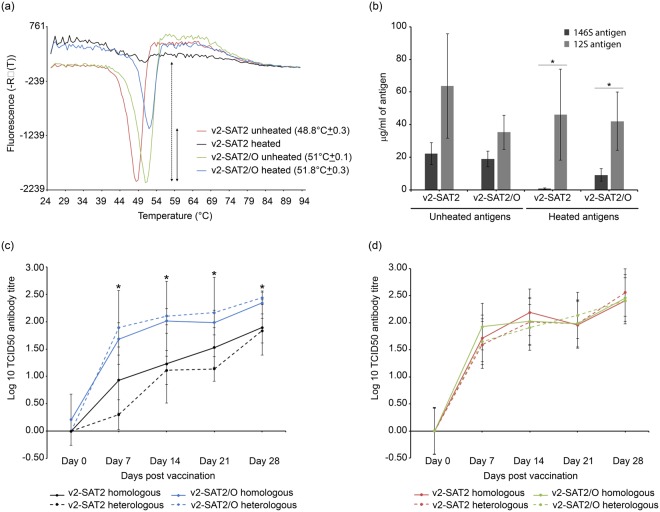
Figure 6.
Assessment of vaccine quality and immunogenicity following exposure to an elevated temperature. (a) Thermostability analysis of v2-SAT2 and v2-SAT2/O vaccines before (unheated) and after (heated) incubation at 45 °C for 2 hours. The respective temperatures of genome release, and hence capsid disassembly, for three independent experiments are shown in brackets. The reduction in fluorescence signal due to loss of intact capsid (146S) is represented by dashed and non-dashed arrows for heated v2-SAT2 and v2-SAT2/O, respectively. (b) Analysis of v2-SAT2 and v2-SAT2/O vaccines before and after incubation at 45 °C for 2 hours by double antibody sandwich ELISA using a single-domain llama antibody (M377F VHH) that specifically recognises 146S. Asterisks indicate a significant decrease (Tukey’s method; 95% confidence, P < 0.05) in the respective 146S:12S vaccine composition following heat treatment. (c,d) Groups of five calves were vaccinated with the respective vaccine at days 0 and boosted on day 21; blood samples were assayed at 0, 7, 14, 21, and 28 days post vaccination. Homologous and heterologous VNT results are shown. Error bars indicate standard deviation. (c) Mean virus neutralizing-antibody titers (VNT (log10)) of calves vaccinated with heat-treated (45 °C for 2 hours) v2-SAT2 or v2-SAT2/O vaccines. Asterisks indicate a significant difference (Tukey’s method; 95% confidence, P < 0.05) in neutralising titres between vaccinate groups. (d) Mean virus neutralizing-antibody titers (VNT (log10)) of calves vaccinated with unheated v2-SAT2 or v2-SAT2/O vaccines.