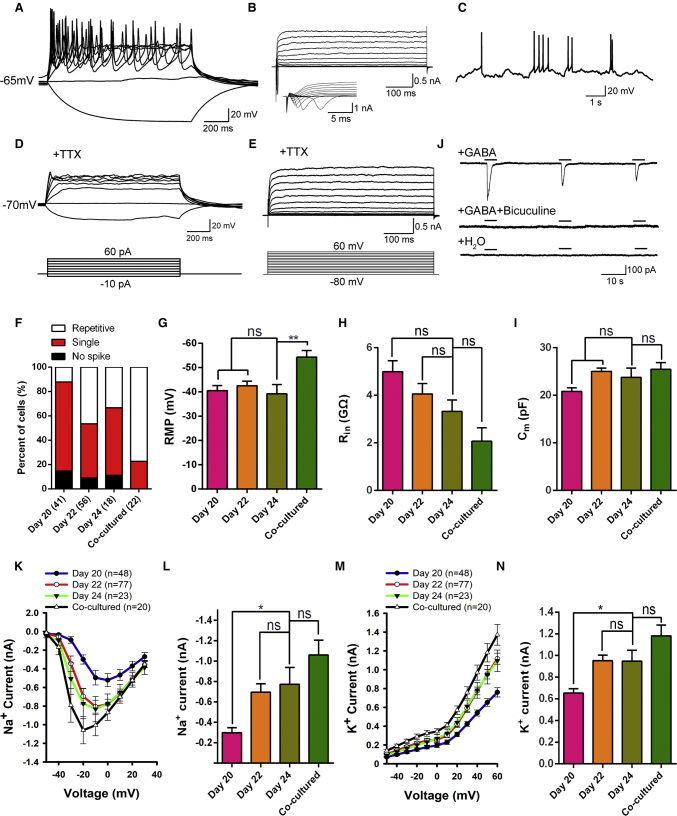
Figure 4.
Electrophysiological Properties of GABAergic MSNs Differentiated from hESCs
(A) Representative traces of membrane potential responding to step depolarization by current injection steps from −10 pA to +60 pA in 10-pA increments. Membrane potential was current-clamped at around −65 mV.
(B) Representative traces of whole-cell currents in voltage-clamp mode; cells were held at −70 mV; step depolarization from −80 mV to +60 mV at 10-mV intervals was delivered. The inset shows Na+ currents.
(C) Spontaneous action potentials (APs) recorded from neurons of 24 days differentiation. No current injection was applied (n = 21).
(D) TTX blocked the membrane APs (n = 47).
(E) The Na+ currents of neurons were blocked by TTX. When the cells were treated with 1 μM TTX, the channel of Na+ currents was blocked completely (n = 47).
(F) Percentages of different AP spikes at 20, 22, and 24 days after differentiation. Increased complexity of AP spikes of differentiated cells over the maturation process. The majority of cells showed single spikes at 24 days differentiation, whereas a larger percentage of cells generated repetitive APs upon co-culture with human glia.
(G–I) Quantification of resting membrane potential (RMP, G), membrane resistance (Rin, H) and membrane capacitance (Cm, I) in neurons at 20, 22, and 24 days after differentiation. Error bars indicate ±SEM. ns, not significant. ∗∗p < 0.01; one-way ANOVA followed by Tukey's multiple comparisons tests.
(J) Focal application of GABA elicited inward membrane currents (n = 19), which was almost completely eliminated by bicuculline. Focal application of ddH2O could not induce inward membrane currents (n = 11).
(K–N) Averaged (means ±SEM) current-voltage relationship (I-V curves) for Na+ and K+ currents, recorded from hESC-derived neurons. ns, not significant. ∗p < 0.05; one-way ANOVA followed by Tukey's multiple comparisons tests.